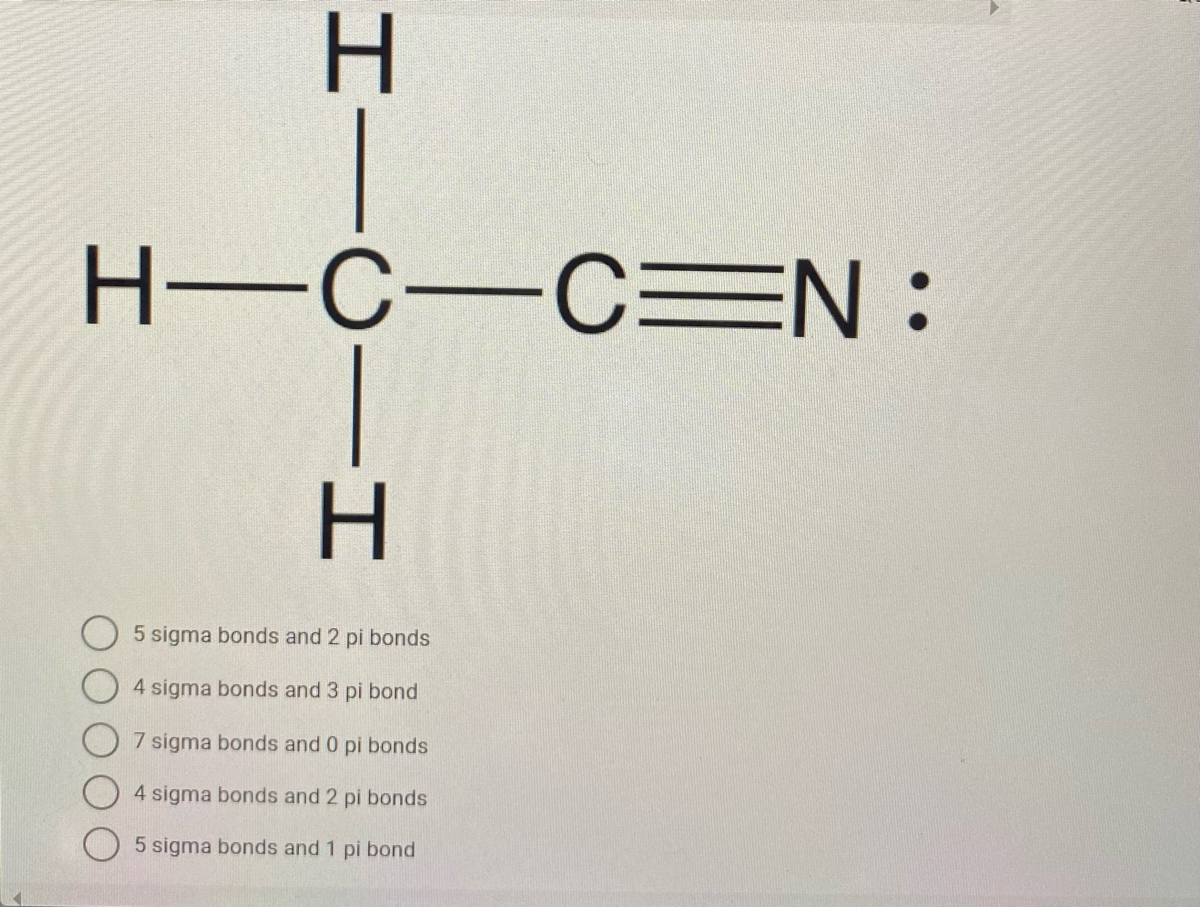
38 so2 pi bonds
radtour-zelt.de 22.05.2022 · The types of intermolecular forces present in ammonia, or NH3, are hydrogen bonds. 2 M NH4Br 62. 2) Of the five major types of crystalline solid, which does each of the following form? Xe. Store. Simple estimates of the effective volume fractions of non-aqueous, polymerically stabilized silicon nitride suspensions gave a reasonable correspondence … Which of the following molecules is having 2ppi - 3dpi bond? P pi p pi bond in a SO2. Open in App. Solution. Verified by Toppr. Solve any question of The p-Block Elements with:-Patterns of problems > Was this answer helpful? 0. 0. Similar questions.
The number of dpi - ppi bonds present respectively in SO2 ... The number of dπ−pπ bonds present respectively in SO 2 ,SO 3 ,ClO 4− are: A 0, 1, 2 B 1, 2, 3 C 2, 3, 4 D 2, 3, 3 Medium Solution Verified by Toppr Correct option is B) The number of dπ−pπ bonds present in SO 2 ,SO 3 ,ClO 4− are 1,2,3 respectively. Video Explanation Was this answer helpful? 0 0 Similar questions
So2 pi bonds
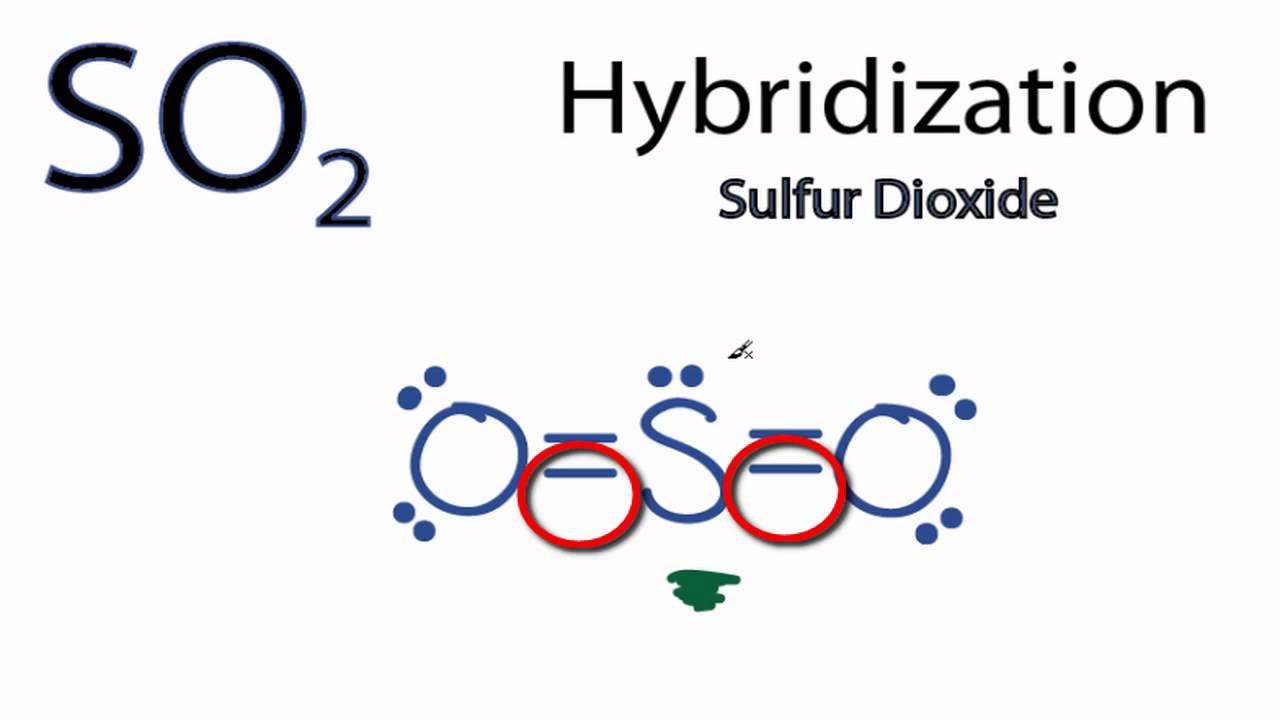
Ch 8 & 9 Flashcards | Quizlet Which of the following types of molecules has a dipole moment (when polar bonds are present)? A) linear molecules with two identical bonds B) tetrahedral molecules (four identical bonds equally spaced) C) trigonal pyramid molecules (three identical bonds) D) trigonal planar molecules (three identical bonds equally spaced) E) None has a dipole ... SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur. SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY The Lewis Structure for SO 2 is a central Sulfur double bonded to each of the Oxygen atoms. A double bond consists of one sigma bond and one pi bond so in total you would have two sigma bonds and two pi bonds (one sigma and pi for one bonded Oxygen and another sigma and pi for the other). Top Sebastian Lee 1L Posts: 157
So2 pi bonds. SO2 Hybridization - YouTube A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. It's also sp2 fo... no of p pi p pi and p pi d pi bonds in so2 - askIITians There are 3 (Pπ-Pπ ) & 1 (Pπ-dπ) Bonds In SO2 Molecule. Because 3 orbitals of P-subshell of S Overlap With 3 Orbitals of P-subshell of O2 & 1 Orbital of d-subshell of S Overlap With 1 orbital of P-subshell of O2........ Hence Proved......🙌🏻 ️ Provide a better Answer & Earn Cool Goodies See our forum point policy sp² hybridized orbitals and pi bonds (video) | Khan Academy For a carbon with 1 double bond and 2 single bonds, the orbitals will become 33% "s" and 66.7% "p" making it "sp2." If there is a triple bond and a single bond, the orbitals will adjust again to become 50% "s" and 50% "p." So to summarize - You can find sp3 bonding when a carbon has 4 single bonds. How does SO4 2- ion form a pπ-dπ bond? - Quora σ bonds= no. of surrounding atoms π bonds= (no. of oxygen atoms) - (no. of negative charge) Hybridization = σ bonds + no. of lone pairs Now, no. of pπ-pπ bonds= no. of unhybridised p orbitals left pπ-dπ bonds will be formed when π bonds are more than the no. of unhybridised p orbitals left.
SO2 Polar or Nonpolar - What's Insight The SO2 molecular geometry is bent, with a bond angle of 120°. ... Between sulfur and each oxygen atom, one sigma and one pi bond are formed. To reduce repulsions, the double bonds and the lone pair are separated as much as possible, and so the molecule is bent. Is SO2 a triple bond? - Answers SO2 contains polar sulfur-oxygen bonds, but no compound, such as SO2, is itself a bond at all. What kind of bond is SO2? Bonds in SO2 are covalent.Bonds between SO2 are dipole-dipole. Is SO2 an... Identify the Type( p pi) (p pi) or (p pi) (d pi) bonds and ... 14149 Points. If one atom has vacant d orbital and other atom has free electrons in the form of lone - pairs then, those lone pairs can be donated to the vacant d orbitals and the bond formation would be called as: p-pi d-pi bond. In SO3 all are p-pi bonds perhaps there is no lone pair left in the S atom to donate. How many pi bonds are in SO_2? | Socratic There are two pi bonds in a single "SO"_2 molecule. First, let us consider the structure of the "SO"_2 molecule: As you can see, the molecule is bent / v-shaped / angular, and there are three regions of electron density: two "O = S" double bonds and a lone pair of electrons. Now, recall that the composition of a double bond is as follows: 1x sigma bond 1x pi bond Therefore, with two double ...
How many pi bonds are in SO2 class 11 chemistry CBSE Sigma and pi bonds are among the types of covalent bonds that can be distinguished by the type of overlap between 2 atomic orbitals. Covalent bond is generally formed by overlapping of orbitals (or we may say by sharing electron pairs). -Sigma Bond ( σ ): These are the strongest type of covalent bond because they are formed by h... Read More What is a p-pi p-pi bond in chemistry? - Quora Answer (1 of 7): If there is bonding between two atoms where one atom is having one vacant orbital and another is having one lone pair of electrons, then if this electron pair is donated to that respective vacant orbital then the bonding is called p(pi) - p(pi) or p(pi) - d(pi) depending on the o... SO2 Ionic or Covalent?| Simple Explanation - What's Insight Sulfur dioxide (SO2) is a covalent molecule. The molecular geometry of sulfur dioxide is a bent shape. The sulfur dioxide molecule has two double bonds between the Sulfur atom and Oxygen atoms. There are 5 lone pairs of electrons in the molecule of SO2. SO2 gives a weak acid solution when dissolved in water. Orbital Hybridization: sp1, sp2, and sp3 Hybridization 01.08.2021 · The number of sigma bonds formed by sulfur atom = two. As it is bonded to two oxygen atoms. Step3: Calculate number of lone pairs . The number of lone pairs on sulfur atom is = (v – b – c) / 2 = (6 – 4 – 0) / 2; Thus 1. Number of valence electrons in sulfur is 6. Total number of bonds including sigma and pi bonds is = 4.
How many pi bond in so2? - Answers How many pi bonds does SO2 have? There are two double bonds.So there are two pi bonds. Is SO2 a polar bond? SO2 contains polar sulfur-oxygen bonds, but no compound, such as SO2, is itself a bond at...
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab Patra ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Writing Formulas and Equations (LaTeX) - Gradescope Help Center Bonds; Precipitate and Gas; All Articles Assignment Workflow. Writing Formulas and Equations (LaTeX) Writing Formulas and Equations (LaTeX) Updated 3 months ago. Math Notation. Greek Letters; Superscripts / Subscripts; Brackets; Matrices; Chemical Formulae. Charges & Isotopes; Reaction Arrows; Bonds; Precipitate and Gas; LaTeX can be used for math symbols in rubric …
70. How many pi bonds form around the central atom in | Chegg.com How many pi bonds form around the central atom in SO2. 71. One s orbital from one atom and one s orbital from another atom are combined to form molecular orbitals for the joined atoms. How many MO's will result from this combination? 72. Consider the MO depicted from the 2p atomic orbitals F2 True or false, we expect mixing of the sand p ...
inorganic chemistry - Why are the two bonds in sulphur dioxide ... The sulfur-oxygen bond has a bond order of 1.5 and does not invoke d orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. ( Wikipedia) However, there is a molecular orbital approach which seems to give a deeper appreciation of the bonding. The picture is this:
isibaba.de 22.05.2022 · There are two secondary types of covalent bonds that are relevant to biology — polar bonds and hydrogen bonds. 2O), which has the following Lewis structure: The bond polarities are shown by the arrows. This makes the bond a polar bond, meaning that it has a positive and negative pole. In chemistry, a polar bond is a type of covalent bond between two …
no. of p pi d pi bonds in so3 2- - Brainly.in Answer: Explanation: If one atom has vacant d orbital and other atom has free electrons in the form of lone - pairs then, those lone pairs can be donated to the vacant d orbitals and the bond formation would be called as: p-pi d-pi bond. In SO3 all are p-pi bonds perhaps there is no lone pair left in the S atom to donate.
Is SO2 have ppi -dpi bond | EduRev NEET Question In SO2 there are one p-pi and one p-pi-dpi bond Top Courses for NEET. Biology Class 11; Biology 31 Years NEET Chapterwise Solved Papers; NEET Mock Test Series; NCERTs for NEET; Biology Class 12. Related Content. Pi bonds and sp2 hybridized orbitals - Chemistry, Class 12; Video | 14:13 min. Sigma & pi bond - Chemical Bonding & Molecular ...
Number of P pi - D pi bonds in So2 and SO3 - YouTube #NEETCHEMISTRY #IITJEECHEMISTRY Number of P pi - D pi bonds in So2 and SO3 .....Clear explanation in a simple manner which is useful for Competitive exami...

Post a Comment for "38 so2 pi bonds"