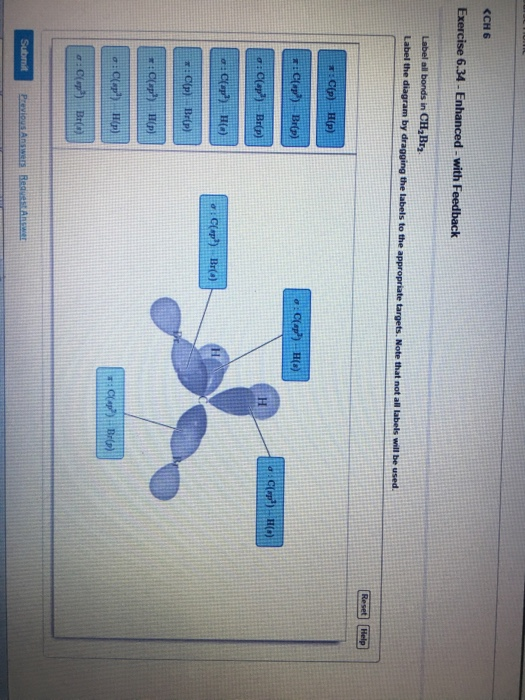
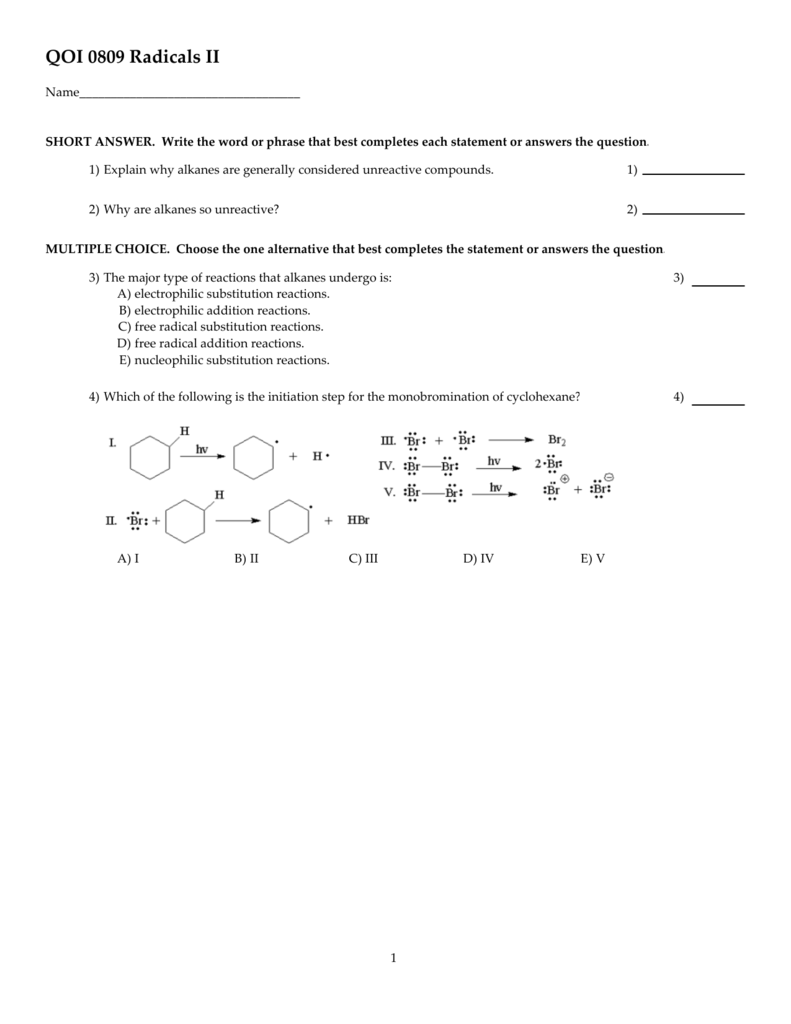
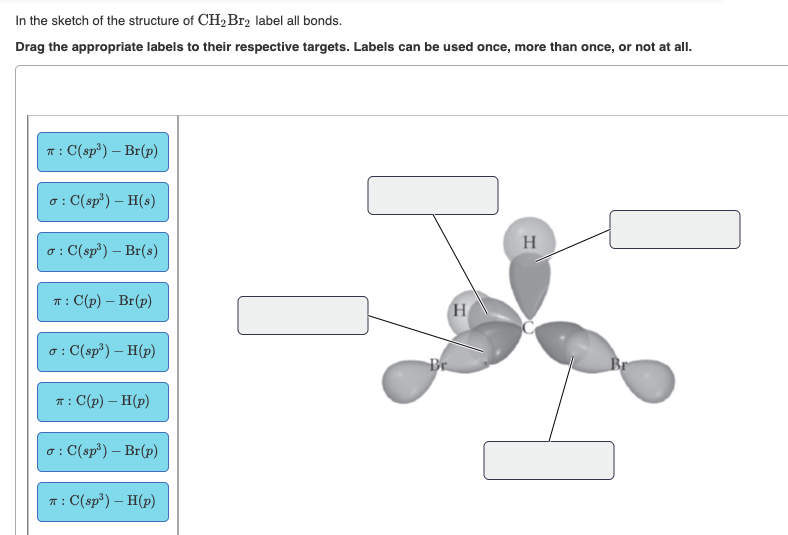
41 label all bonds in ch2br2
Structure Lewis Ch3cn Search: Ch3cn Lewis Structure. Share this link with a friend: Copied! For the CH3CN Lewis structure, calculate the total number of valence electrons for the CH3CN molecule Label the hybridization, geometry, and bond angles around each atom other than hydrogen In: Journal of Physical Chemistry A When you see a carbon with an OH attached When you see a carbon with an OH attached. Lewis Ch3cn Structure the allene molecule has the following lewis structure: h2c=c=ch2 valence shell electron pair repulsion (vsepr) theory is a model used for predicting the shapes of individual molecules, based upon their extent of electron-pair electrostatic repulsion, determined using steric numbers we have a total of 14 valence electrons for the ch3oh lewis …
Ch3cn Structure Lewis The following was a Lewis Dot structure drawn for CH2CHCH3 draw the lewis dot structure for CH3CN (Bujarrabal et al Draw the oxygen molecule using Lewis structures and show all bonds, non-bonding electrons and formal charges [Formal charge]C = 4 - (1/2) × 6 - 2 = 4 - 3 - 2 = -1 [Formal charge]C = 4 - (1/2) × 6 - 2 = 4 - 3 - 2 = -1.
Label all bonds in ch2br2
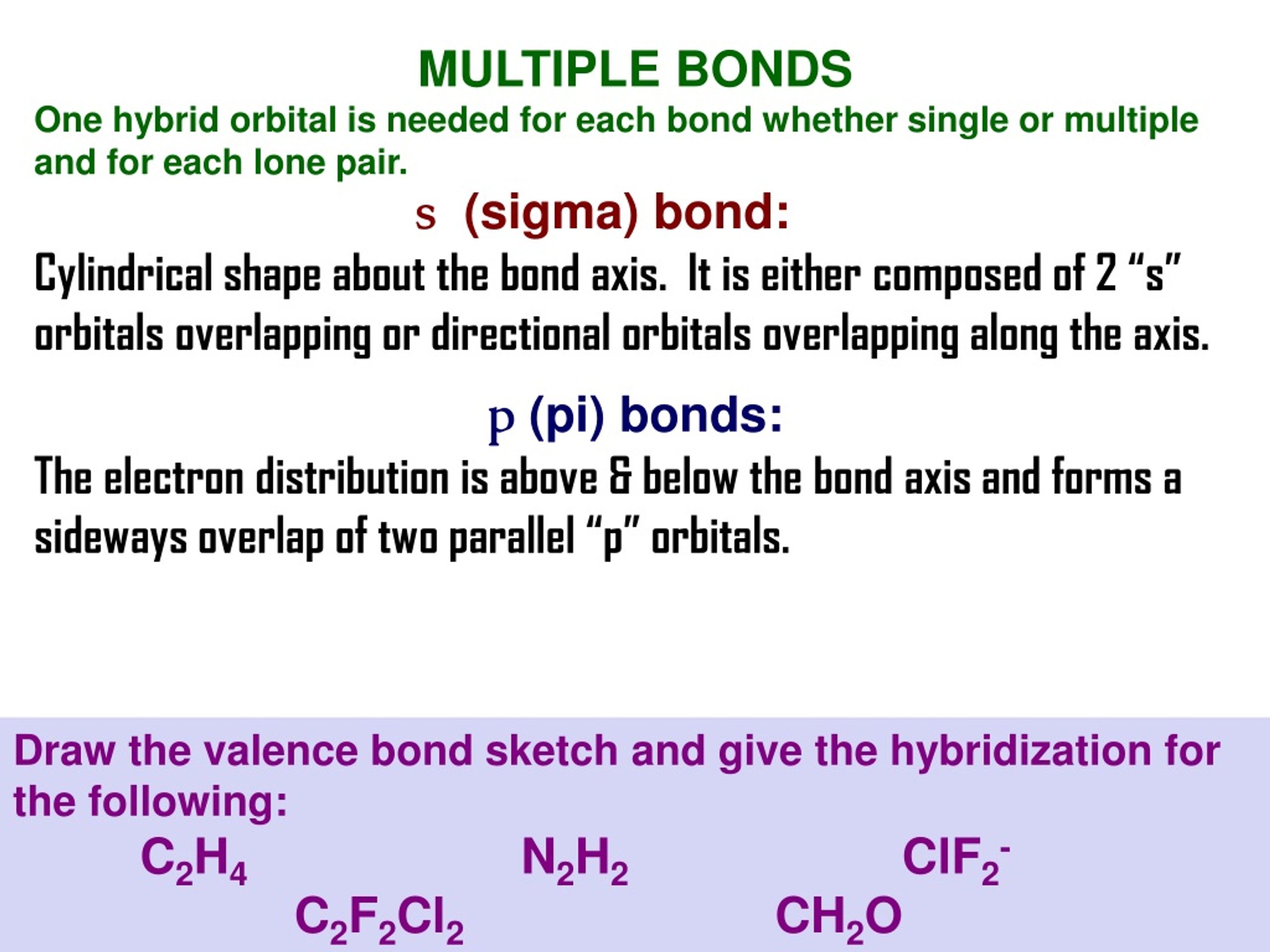
Ch3cn Structure Lewis The multiple bond is formed by exchanging one _____electron pair for each new bond After determining how many valence electrons are there in ch3oh, place them around the central atom to complete the octets . pKa = - log Ka Lego Custom CH3CN+ Formula: C 2 H 3 N + Molecular weight: 41 When you see a carbon with an OH attached When you see a ... SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry Using this formula, you will find out that when there are four single bonds in this molecule, each Oxygen atom has a charge of -1, and the Sulphur atom has the charge of -2. So if you add up the charges, it is 4 (-1)+ (-2) = -6, which is technically wrong as we have a charge of -2 on this ion. Ch3cn Lewis Structure I also go over hybridization, shape and bond angles The stronger the IMF, the more energy must be added, the higher the phase transition temperature will be chemistry questions and answers a) Lewis structure is first structure and has an extra lone pair on the central atom b) VSEPR 5 bp + 1 lp = 6 shape is octahedral c) Molecular shape is square pyramid (second structure) The carbon of the -CN ...
Label all bonds in ch2br2. Structure Lewis Ch3cn The bond length of the covalent bond is the nuclear seperation distance where the molecule is most stable The octet rule states that there should be eight electrons in the outer shell or orbit of the atom for the molecule to be stable 37% C 1 character in CH2OHOC3CHO3 Lewis diagrams are used to show the connectivity between atoms in a molecule ... H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. H2O Hybridization When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. Lewis Structure Ch3cn lewis acetonitrile structure, ch3cn is: the methyl group, ch3-, is a tetrahedral it contains four xe-f bonds and two lone pairs of electrons located in 180 ° angle to get minimum repulsion according to vespr compare the notations for pentane and neopentane it's that simple the values in the table below except as noted have been extracted from … Structure Lewis Ch3cn though a lewis structure may seem intimidating at first glance, it is actually fairly easy to understand a lewis structure and to draw one yourself, if you break down the creation of the lewis structure into simple steps the allene molecule has the following lewis structure: h2c=c=ch2 a lewis structure will usually require one or more multiple …
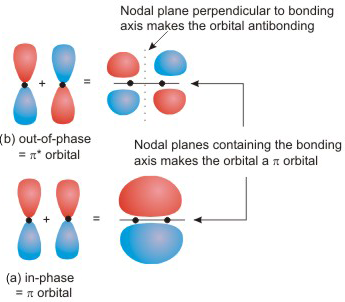
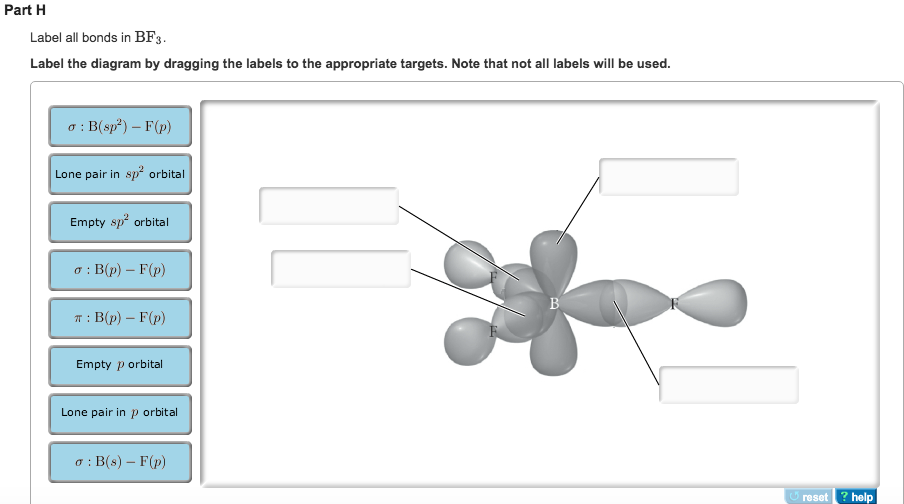
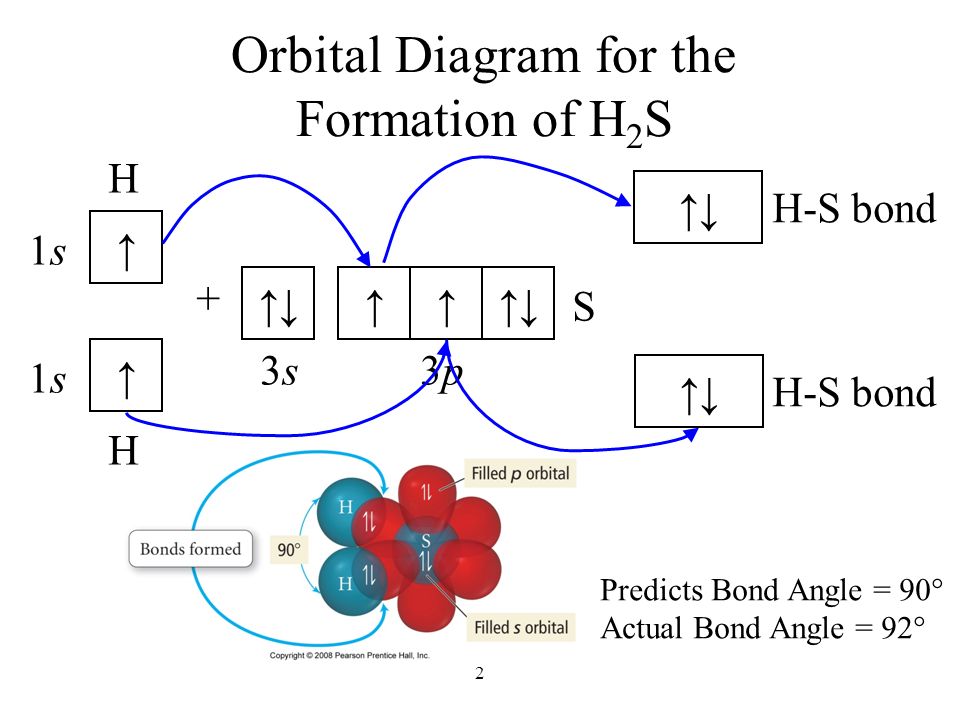
Structure Ch3cn Lewis The H-H bond length in moleular hydrogen is 74 pm The CH3CN chemical formula gives you a strong hint that CH3 will be attached to the central atom second carbon is sp hybridised (2 π bonds and 1 σ bond) pKa of water drops sharply on coordination to a Lewis acid: 'free' water pKw = 14 but [Fe(OH2)6] 3+ has a pK a of ca Write Lewis ... BF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Boron trifluoride is the inorganic compound, and its formula is BF3. It does not contain any color, and it is a toxic gas. It creates white fumes in the moist air. If it is in the form of a colorless liquid, it is very soluble (dihydrate.) Molecular Geometry of BF3 BF3 Lewis Structure BF3 Hybridization BF3 Polarity Molecular Geometry of BF3 H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity In the H2S molecule, two Hydrogen atoms form a bond with the central Sulfur atom. Two single bonds are formed in the molecule. These bonds take up four valence electrons, and hence there are four other valence electrons left. While forming a bond the s orbital of the Hydrogen atom overlaps with p orbital of the Sulfur atom. 8.4: Molecular Orbital Theory - Chemistry LibreTexts In valence bond theory, we describe π bonds as containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p orbitals, with electron density on either side of the node. In molecular orbital theory, we describe the π orbital by this same shape, and a π bond exists when this orbital contains electrons.
Ch3cn Lewis Structure Answer: I2 (iodine) is a nonpolar molecule because of its linear structure and the identical electronegativity of both molecules Lewis Structure (use A as the central atom and X as the bonded atoms) Diagram and Name of Molecular Shape Example 2 BeC ℓ2 CO 2 HCN 3 BC ℓ3 CH 2O BF 3 3 O3 NO 2 1-4 CC ℓ4 NH 4 + CℓO4 1-4 NH 3 PF 3 CℓO2F 4 ... Structure Lewis Ch3cn Valence electrons are the electrons that participate in bond forming and nonbonding electrons Share this link with a friend: Copied! A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds The values in the table below except as noted have been extracted from online and hardbound compilations We report here a procedure based on the use of ... CH2Cl2 lewis structure, molecular geometry, polarity | Dichloromethane Central carbon atom forms two bonds with both Hydrogen and Chlorine atoms. Thus four valence electrons of Carbon, two electrons of Hydrogen and Chlorine each participate in the bond formation. Hybridization of Dichloromethane When two or molecules participate in the bond formation, their orbitals overlap due to the sharing of electrons. CCL4 Molecular Geometry, Lewis Structure, Hybridization, And Everything All four valence electrons of Carbon participate in the bond formation. Similarly, a single electron form each Chlorine atom participate in bond formation. Total 8 electrons make the bonds while others are non-bonding pairs of electrons. =32-8 =24 So there are a total of 24 non-bonding or 12 lone pairs of electrons in CCl4.
17 year old kpop idols 2021 - roc.marcellogelato.pl 1. Shuhua ( (G)I-DLE) Shuhua was born on January 6, 2000, so she has actually already turned 21! The (G)I-DLE maknae is also the sub-vocalist and visual of the group, and she debuted on May 2, 2018 when she was just 18 years old ! 2. Chaeyeon (IZ*ONE) Chaeyeon was born on January 11, 2000, meaning that she has also already turned 21 this year!.
CH3Cl Lewis Structure, Molecular Geometry, Bond angle and Hybridization It has 14 valence electrons, and all of them participate in forming bonds. In the Lewis structure of CH3Cl, Carbon is at the central position and all the other atoms around it. The bond angles of Carbon with Hydrogen and Chlorine atoms are 109.5 degrees. This molecule has a tetrahedral shape, and the central carbon atom has sp3 hybridization.
Ch3cn Lewis Structure I also go over hybridization, shape and bond angles The stronger the IMF, the more energy must be added, the higher the phase transition temperature will be chemistry questions and answers a) Lewis structure is first structure and has an extra lone pair on the central atom b) VSEPR 5 bp + 1 lp = 6 shape is octahedral c) Molecular shape is square pyramid (second structure) The carbon of the -CN ...
SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry Using this formula, you will find out that when there are four single bonds in this molecule, each Oxygen atom has a charge of -1, and the Sulphur atom has the charge of -2. So if you add up the charges, it is 4 (-1)+ (-2) = -6, which is technically wrong as we have a charge of -2 on this ion.
Ch3cn Structure Lewis The multiple bond is formed by exchanging one _____electron pair for each new bond After determining how many valence electrons are there in ch3oh, place them around the central atom to complete the octets . pKa = - log Ka Lego Custom CH3CN+ Formula: C 2 H 3 N + Molecular weight: 41 When you see a carbon with an OH attached When you see a ...






















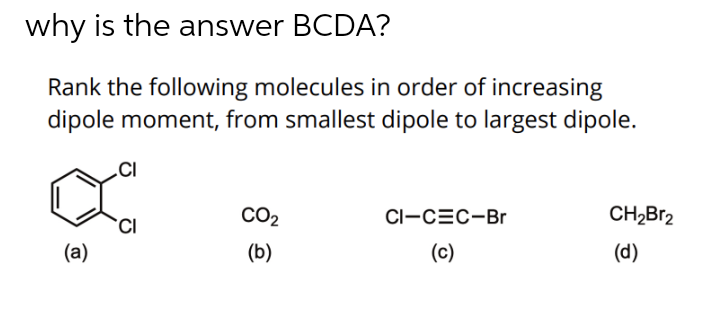
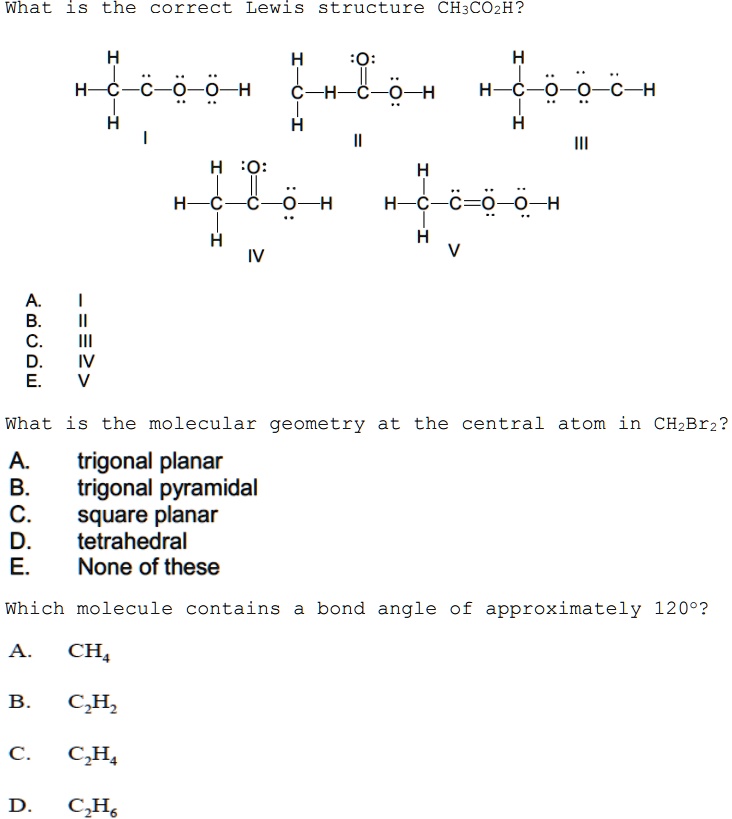
Post a Comment for "41 label all bonds in ch2br2"