43 namenda off-label uses
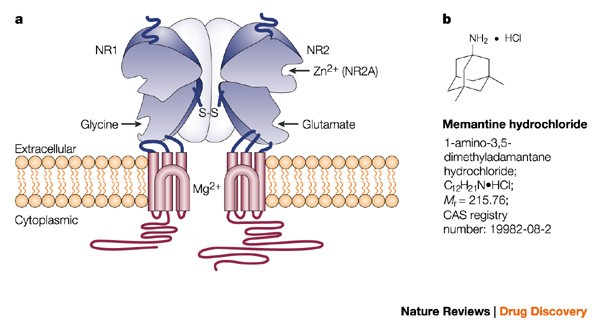
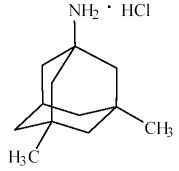
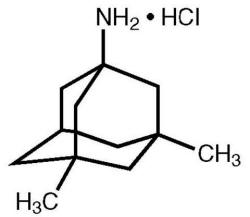
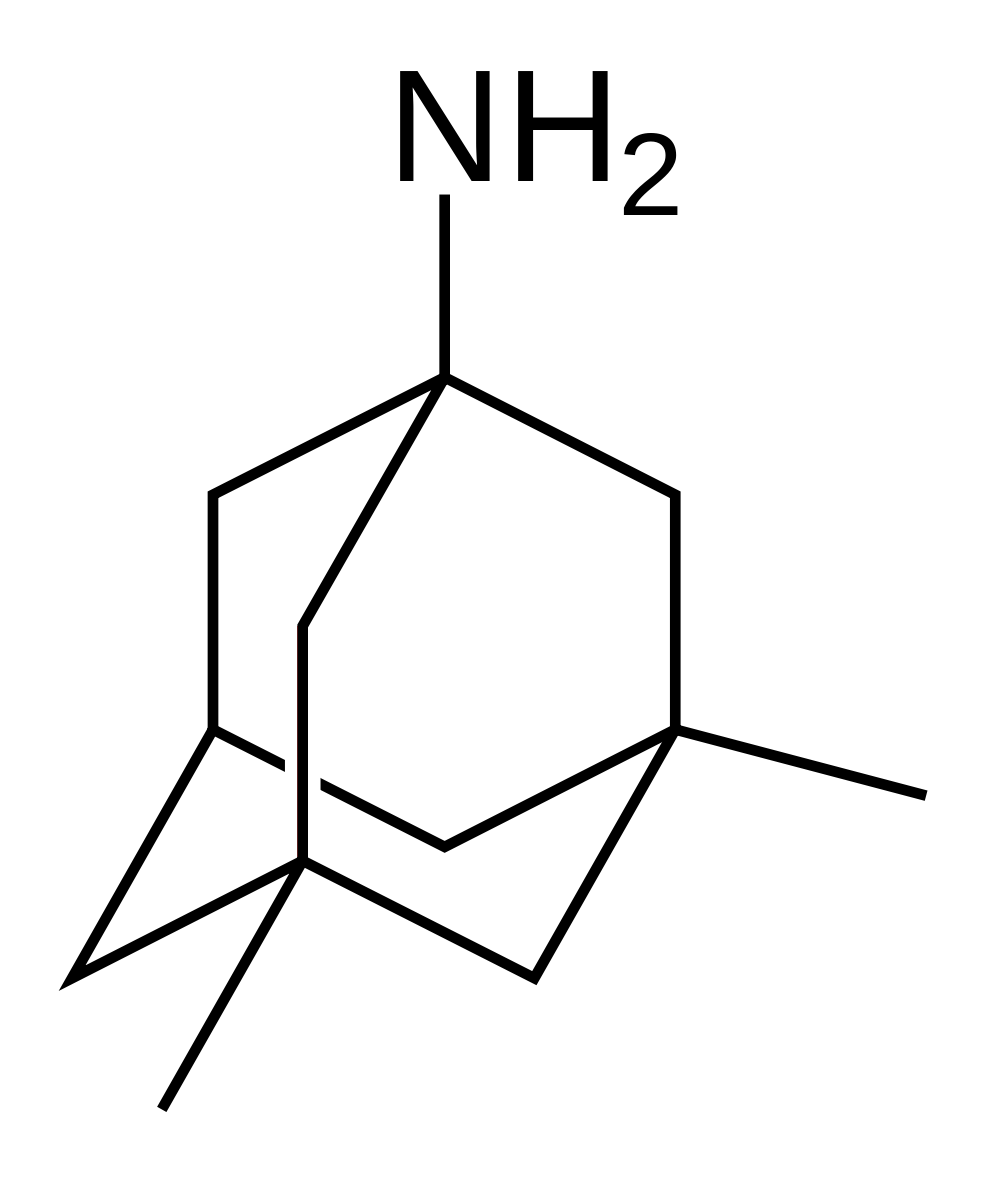
Memantine (Oral Route) Description and Brand Names - Mayo Clinic Namenda XR; Namenda XR Titration Pack; Descriptions. Memantine is used to treat moderate to severe Alzheimer's disease. Memantine is not a cure for Alzheimer's disease but it can help people with the disease. Memantine will not cure Alzheimer's disease, and it will not stop the disease from getting worse. Memantine Monograph for Professionals - Drugs.com Memantine (Monograph) Brand name: Namenda. Drug class: Central Nervous System Agents, Miscellaneous. VA class: CN900. Chemical name: 3,5-Dimethyl-1-adamantanamine21. Molecular formula: C 12 HN. CAS number: 19982-08-2. Medically reviewed by Drugs.com on Oct 10, 2022. Written by ASHP.
Namenda Oral: Uses, Side Effects, Interactions, Pictures ... - WebMD Uses. Memantine is used to treat moderate to severe confusion ( dementia) related to Alzheimer's disease. It does not cure Alzheimer's disease, but it may improve memory, awareness, and the ...

Namenda off-label uses
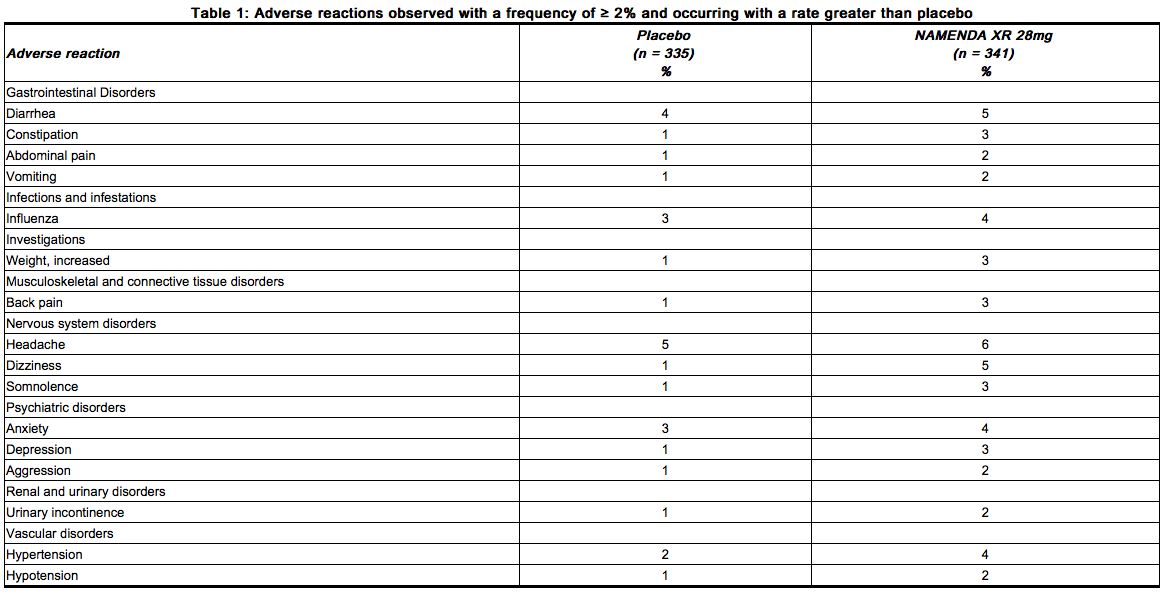
namenda off label uses - MedHelp Though there is a good theoretical basis for the use of arimidex on endometriosis, I believe that this is still an off-label indication for the drug and the clinical basis for its use is still from phase II clinical trials (studies with still low number of patients). Until such time that the indication of endometriosis can be demonstrated in large phase III clinical trials, the use of arimidex ... PDF HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ... NAMENDA XR in the controlled clinical trial, defined as those occurring at a frequency of at least 5% in the NAMENDA XR group and at a frequency higher than placebo, were headache, diarrhea and dizziness. Table 1 lists adverse reactions that were observed at an incidence of ≥ 2% in the NAMENDA XR group and occurred at a rate greater than placebo. Namenda XR (memantine) dosing, indications, interactions, adverse ... Mild-to-Moderate Vascular Dementia (Off-label) 5 mg (immediate-release) PO qDay; titrate by 5 mg q7days to target dose 10 mg twice daily. Not indicated. Next: Interactions. ... Namenda XR. USES: Memantine is used to treat moderate to severe confusion (dementia) related to Alzheimer's disease. It does not cure Alzheimer's disease, but it may ...
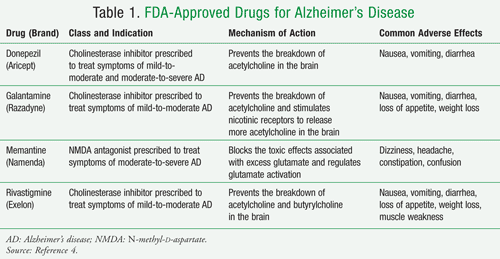
Namenda off-label uses. Off-Label Use of Alzheimer's Drugs - U.S. Pharmacist Off-Label Use of Alzheimer's Drugs. US Pharm. 2012;37 (3):61-63. For those who have lost the attachment to a loved one's mind suffering from Alzheimer's disease, there is hardly anything more devastating. This spouse, mother, father, sister, brother, or friend that you have known and loved slowly but distinctly loses touch with reality ... Namenda only Works for Severe Alzheimer's Disease and Dementia Namenda (also called Memantine) was approved by the FDA in 2003 for use in people with "moderate to severe" Alzheimer's disease or other types of dementia. The FDA rejected the manufacturer's application to expand approval to include mild Alzheimer's or dementia. [1] However, the drug is often prescribed "off-label" for patients ... Off-Label Anti-Anxiety Medication: Types, Risks, and Safety - Psych Central Memantine (Namenda) ... Maher AR, et al. (2011). Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: ... A systematic review of off-label uses of memantine for psychiatric ... Recent data points to glutamatergic dysfunction in mood disorders, anxiety disorders, obsessive-compulsive disorder, and schizophrenia. Memantine, a drug approved by the FDA for the treatment of moderate to severe Alzheimer's disease that acts at the N-methyl-d-aspartate receptor, has been used off-label for various psychiatric disorders.
12 Curious and Unexpected Off-Label Drugs - Suzy Cohen Many drugs are used for off-label purposes. Find out if you're taking a drug like that. Medications aren't always used for what they were intended for. Many drugs are used for off-label purposes. ... Namenda (Memantine). This is a popular medication that the FDA approved in 2003, to treat Alzheimer's disease. But practitioners all over the ... All About Memantine: Approved & Unofficial Uses + Side-Effects Off-Label Medical Uses. Occasionally, doctors will prescribe drugs like memantine to help treat conditions that fall outside of the official uses approved by the FDA - also known as "off-label" drug use [9, 10, 11]. Usually, this is done because there is actually decent evidence that the drug may help, although this evidence might not be ... Namenda: Package Insert - Drugs.com The molecular formula is C12H21N•HCl and the molecular weight is 215.76. Memantine hydrochloride occurs as a fine white to off-white powder and is soluble in water. Namenda tablets are available for oral administration as capsule-shaped, film-coated tablets containing 5 mg and 10 mg of memantine hydrochloride. Off-label uses of Memantine (Namenda) - Mortimer, M.D Off-label uses of Memantine (Namenda) Memantine is a NMDA (N-methyl-D-aspartate) glutamate receptor antagonist which is, as one Portland psychiatrist summarized, "…good for any distressed neuron.". For those who discuss the roles of kynurenine and brain-derived neurotrophic factor, and the various functions of astrocytes and ...
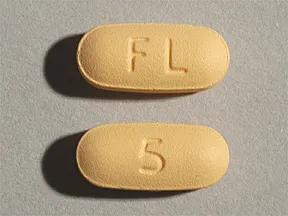
PDF Page 1 of 18 Reference ID: 3394954 - Food and Drug Administration NAMENDA 10 mg tablet: capsule-shaped, film-coated tablets are gray, with the strength (10) debossed on one side and FL on the other. NAMENDA 2 mg/mL oral solution: clear, alcohol-free, sugar-free, and peppermint flavored. 4 CONTRAINDICATIONS . NAMENDA (memantine hydrochloride) is contraindicated in patients with known . Page 2 of 18 PDF Highlights of Prescribing Information ... NAMENDA has not been systematically evaluated in patients with a seizure disorder. In clinical trials of NAMENDA, seizures occurred in 0.2% of patients treated with NAMENDA and 0.5% of patients treated with placebo. 6.2 Postmarketing Experience The following adverse reactions have been identified during post-approval use of memantine. Namenda : Uses, Dosage, Side Effects - Drugs.com seizure (convulsions); or. unusual changes in mood or behavior. Common Namenda side effects may include: diarrhea; dizziness; or. headache. This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. A systematic review of off-label uses of memantine for psychiatric ... Memantine, a drug approved by the FDA for the treatment of moderate to severe Alzheimer's disease that acts at the N-methyl-d-aspartate receptor, has been used off-label for various psychiatric disorders. Although promising, the available data for the use of memantine in these disorders is limited. Given this data, the routine use of memantine ...
Memantine (Nameda): Uses, Side Effects, Dosage & Reviews - GoodRx Typical dosing for memantine (Nameda) Immediate-release (IR) tablets and oral liquid: The typical starting dose is 5 mg by mouth once a day. This dose will be slowly raised over a 4-week period to 20 mg by mouth twice a day. Extended-release (XR) capsules: The typical starting dose is 7 mg by mouth once daily.
3 Uses for Namenda | HelpRx Blog Namenda and Fibromyalgia. A study out of Spain has found that a promising off-label use of Namenda may be treatment of the symptoms of fibromyalgia, a disorder marked by widespread pain, mood and memory problems, and fatigue and loss of sleep. It is believed to be caused by overly-stimulated NMDA receptors.
Namenda XR (memantine) dosing, indications, interactions, adverse ... Mild-to-Moderate Vascular Dementia (Off-label) 5 mg (immediate-release) PO qDay; titrate by 5 mg q7days to target dose 10 mg twice daily. Not indicated. Next: Interactions. ... Namenda XR. USES: Memantine is used to treat moderate to severe confusion (dementia) related to Alzheimer's disease. It does not cure Alzheimer's disease, but it may ...
PDF HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include ... NAMENDA XR in the controlled clinical trial, defined as those occurring at a frequency of at least 5% in the NAMENDA XR group and at a frequency higher than placebo, were headache, diarrhea and dizziness. Table 1 lists adverse reactions that were observed at an incidence of ≥ 2% in the NAMENDA XR group and occurred at a rate greater than placebo.
namenda off label uses - MedHelp Though there is a good theoretical basis for the use of arimidex on endometriosis, I believe that this is still an off-label indication for the drug and the clinical basis for its use is still from phase II clinical trials (studies with still low number of patients). Until such time that the indication of endometriosis can be demonstrated in large phase III clinical trials, the use of arimidex ...























Post a Comment for "43 namenda off-label uses"