39 open-label extension study
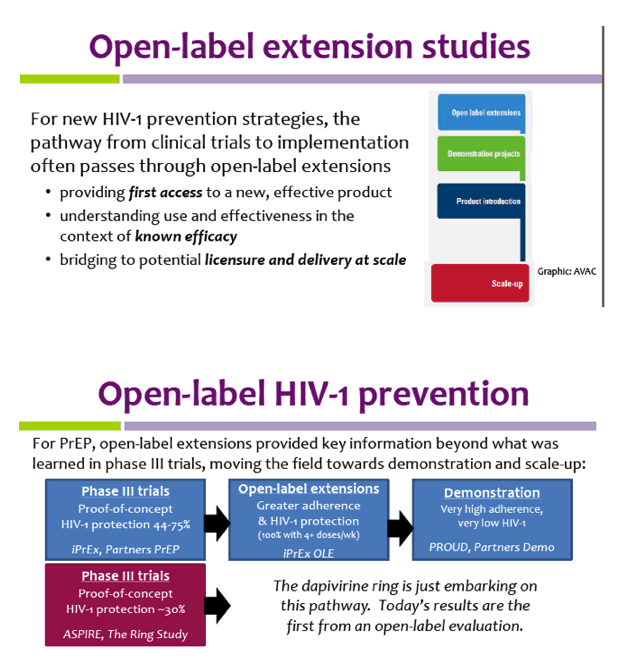
Open-Label Extension Studies | SpringerLink As the name implies, an open-label extension study is an 'appendage' to a randomised controlled clinical trial, usually of an unregistered medicine or intervention. Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation. The open-label extension study is identified formally as a study. Cassava Sciences Announces Initiation of an Open-label Extension Study 13.10.2022 · Alzheimer’s Patients Who Complete Participation in a Phase 3 Study of Simufilam are Eligible to Enroll52-week Study of Simufilam 100 mgUp to 1,600 Patients Are Expected to Enroll AUSTIN, Texas ...
Cassava Sciences Announces Initiation of an Open-label Extension Study Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice...
Open-label extension study
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... There is another type of study that exists between the traditional clinical trial phases and application for approval of a new medication: an open-label extension (OLE) study, sometimes also called a long-term extension study. Consent to open label extension studies: some ethical issues A recent MEDLINE search for "open label extension studies", limited by publication type to randomised controlled trials, produced 55 references between 1992 and 2000. Open label extension studies typically follow on from (and are hence an extension of) a double blind, placebo controlled trial of a new medication. Use Extension Studies To Enhance Phase 3 Data - Clinical Leader An open-label extension study is one that will lie between a double-blind, randomized controlled drug trial (the parent trial) and the request for FDA approval. Jigna Parekh, a senior study manager for inVentiv Health Clinical, has been working in the clinical field for more than 10 years and is currently embedded with Sanofi performing ...
Open-label extension study. argenx Presents Interim Results from ADAPT+ Open-Label Extension Study ... argenx SE (Euronext & Nasdaq: ARGX), a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases, today announced interim results from ADAPT+, an ongoing Phase 3, open-label, three-year extension study evaluating long-term safety, tolerability and efficacy of VYVGART® (efgartigimod alfa-fcab) for the treatment of adults with gMG. Atara Biotherapeutics Presents New MRI and Updated Open-Label Extension ... 26.10.2022 · Atara Biotherapeutics Presents New MRI and Updated Open-Label Extension Data from Phase 1 Study of ATA188 in Progressive Multiple Sclerosis at ECTRIMS 2022. PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology of prolonged open-label extension. For example, the study of prednisolone remained randomized for 2 years2. The safety issues do not constitute a sufficient reason for con-ducting open-label extension studies. The third purpose may be to demonstrate continued effi-cacy of the drug over a longer period of time or to show that Ionis presents positive Phase 2 data from open label extension study of ... carlsbad, calif., nov. 13, 2022 /prnewswire/ -- ionis pharmaceuticals, inc. (nasdaq: ions) today presented positive results from a phase 2 open-label extension (ole) study evaluating the...
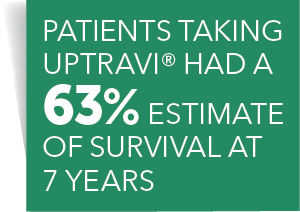
Open Label Extension Study Definition | Law Insider Examples of Open Label Extension Study in a sentence. Long-Term Use of Patisiran, an Investigational RNAi Therapeutic, in Patients with Hereditary Transthyretin- Mediated Amyloidosis: Baseline Demographics and Interim Data from Global Open Label Extension Study.. Funds received by the Company from the exercise of New Options, which are exercisable at any time prior to the Expiry Date, are to ... Open-Label Extension Study | UPTRAVI® (selexipag) HCP In long-term follow-up of patients who were treated with UPTRAVI® in the placebo-controlled study (N=574) and the open-label extension study (N=330, of 574), Kaplan-Meier estimates of survival at 1, 2, 5, and 7 years were 92%, 85%, 71%, and 63%, respectively. The median follow-up duration was 4.5 years, and the median exposure of UPTRAVI® was ... Open label extension studies and patient selection biases Concerns over the scientific validity of open label extension studies to randomized controlled trials have recently been raised. Patients experiencing adverse events will be withdrawn before the follow-on period of the study, and those experiencing milder side-effects will be less likely to opt to continue into the open label extension. Spotlight on Open-Label Extension Studies The stated objective of most OLE studies is to obtain long-term safety and tolerability data. Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study.
Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with ... 28.1.2016 · Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Here, tofacitinib safety and efficacy data from a long-term extension study in Japanese patients are presented. Study A3921041 was a multi-centre, open-label, long-term extension study that included Japanese patients who had participated in a prior Phase 2 or Phase 3 study of … clinicaltrials.gov › ct2 › showStudy of Monovalent and Bivalent Recombinant Protein Vaccines ... May 27, 2021 · A Parallel-group, Phase III, Multi-stage, Modified Double-blind, Multi-armed Study to Assess the Efficacy, Safety, and Immunogenicity of Two SARS-CoV-2 Adjuvanted Recombinant Protein Vaccines (Monovalent and Bivalent) for Prevention Against COVID-19 in Adults 18 Years of Age and Older as a Primary Series and Open-label Extension to Assess ... Open-label Extension Study of Efficacy, Safety and Tolerability of ... Open-label Extension Study of Efficacy, Safety and Tolerability of Secukinumab in Patients With Active Lupus Nephritis The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. clinicaltrials.gov › ct2 › showNonrelapsing Secondary Progressive ... - ClinicalTrials.gov Jun 02, 2020 · Choosing to participate in a study is an important personal decision. Talk with your doctor and family members or friends about deciding to join a study. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. For general information, Learn About Clinical Studies.
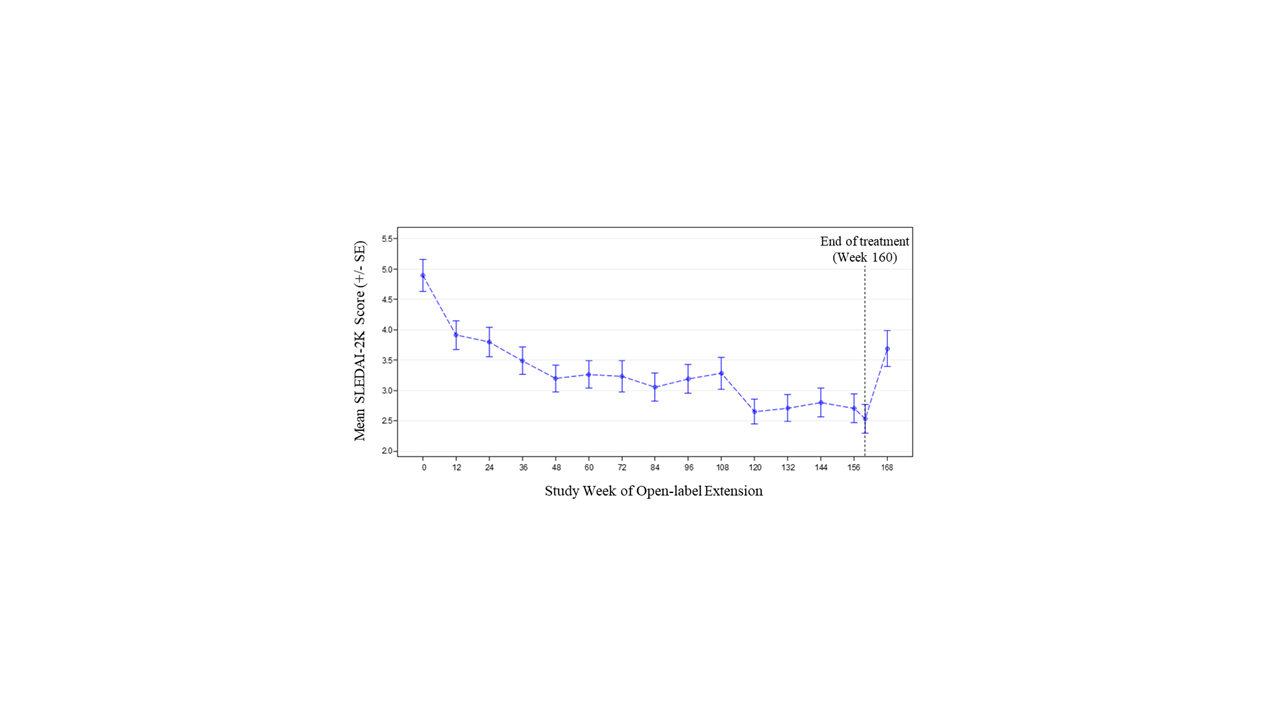
An Open-Label Extension Study of the Safety and Efficacy of Risperidone ... Objective: The purpose of this study was to evaluate the long-term safety and efficacy of risperidone in treating irritability and related behaviors in children and adolescents with autistic disorders. Methods: In this 6 month (26 week) open-label extension (OLE) study, patients (5-17 years of age, who completed the previous fixed-dose, 6 week, double-blind [DB] phase) were flexibly dosed ...
Ionis presents positive Phase 2 data from open label extension study … 13.11.2022 · CARLSBAD, Calif., Nov. 13, 2022 /PRNewswire/ -- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) today presented positive results from a Phase 2 open-label extension (OLE) study evaluating the safety ...
Epilepsia Publishes Interim Results of Open-Label Extension Study … 23.11.2022 · Peer-reviewed results from the Phase 3 open-label extension study of FINTEPLA ® in LGS provide significant reduction in the frequency of multiple seizure types associated with a drop or fall for ...
› news-releases › newsCassava Sciences Announces Initiation of an Open-label ... Oct 13, 2022 · Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice daily over 52 weeks. Each clinical investigational site must choose whether to participate in this open-label extension study. Patient enrollment for ...
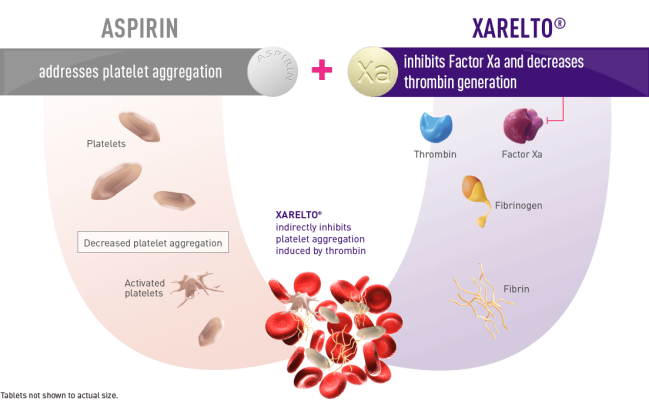
Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
Centessa Pharmaceuticals Announces Additional 18-Months of Continued ... AP-0101 is an ongoing first-in-human open-label multi-center study to investigate the safety, tolerability, pharmacokinetics, and efficacy of subcutaneous doses of SerpinPC in male participants ...
Cassava Sciences Announces Initiation of an Open-label Extension Study ... 13.10.2022 · Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice daily over 52 weeks. Each clinical investigational site must choose whether to participate in this open-label extension study.
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a key...
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... What does "Open Label Extension or OLE" mean? Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo.
Open-label extension studies: do they provide meaningful ... - PubMed Consumers, institutions where these studies are conducted and research ethics committees need to be convinced of the motives, as well as the quality, of the open-label extension study and its execution before supporting such studies. Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines.
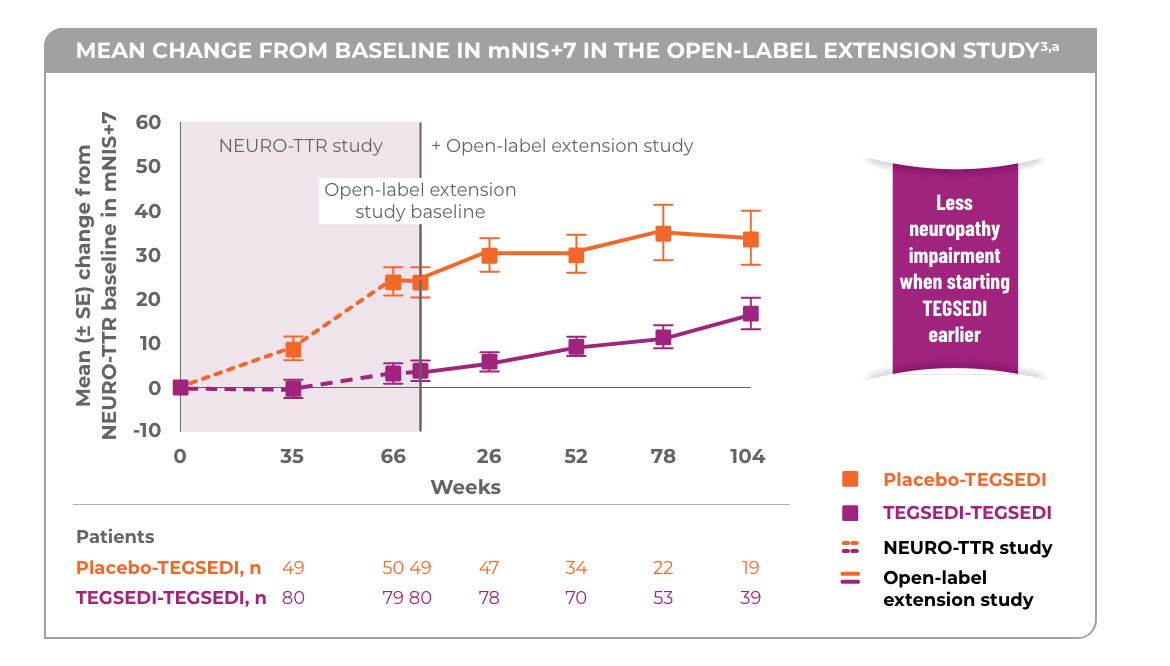
Biogen Announces Topline Results from the Tofersen Phase 3 Study … 17.10.2021 · In addition, a pre-specified integration of data from VALOR and its ongoing open-label extension study (OLE) reinforced these findings and showed that early tofersen initiation led to less decline across multiple measures of motor function, respiratory function, muscle strength, and quality of life in people with SOD1-ALS.
Prolia® (denosumab) Open-Label Extension Study Design | Prolia® The open-label extension study is not blinded, not controlled, and includes inherent self-selection bias. A total of 351 patients (7.7%) had adverse events that led to discontinuation of Prolia ® and 277 patients (6.1%) had adverse events that led to discontinuation of the study. 1,4
› studies › studyClinical Studies and Case Reports - cannabis med An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics: Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT: 2013: Cannabis: Open study
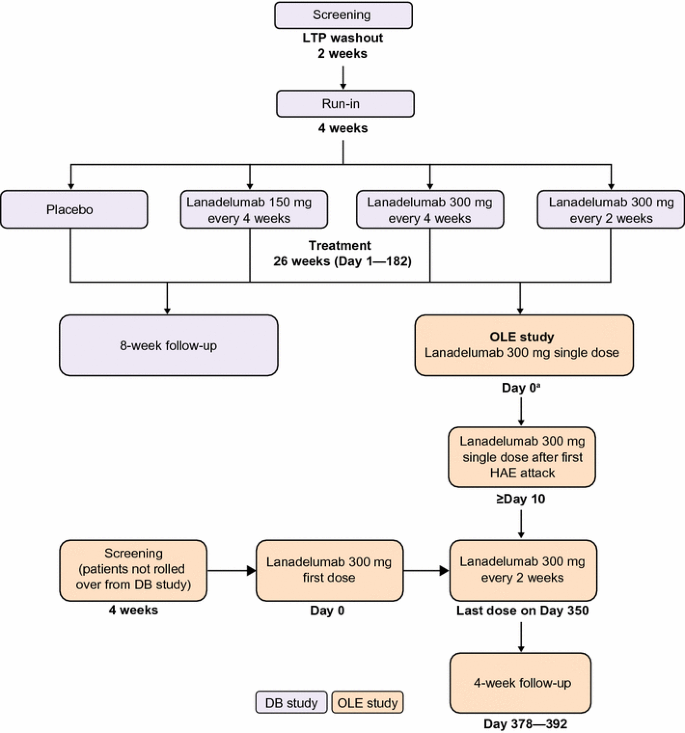
Open Label Extension Study of AMX0035 in Patients With ALS - Full Text ... The Centaur Open Label Extension Study (CENTAUR-OLE) is designed to provide longer term access to AMX0035 for patients with ALS who participated in the CENTAUR study. The study will assess longer term safety and therapeutic potential of AMX0035. Study Design Go to Resource links provided by the National Library of Medicine
Avidity Biosciences Enrolls Patients in the MARINA™ Open-Label ... san diego, aug. 2, 2022 /prnewswire/ -- avidity biosciences, inc. (nasdaq: rna ), a biopharmaceutical company committed to delivering a new class of rna therapeutics called antibody oligonucleotide...
› news-releases › epilepsiaEpilepsia Publishes Interim Results of Open-Label Extension ... Nov 23, 2022 · Peer-reviewed results from the Phase 3 open-label extension study of FINTEPLA ® in LGS provide significant reduction in the frequency of multiple seizure types associated with a drop or fall for ...
Cassava Sciences Announces Initiation of an Open-label Extension Study Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice daily over 52 weeks. Each clinical investigational site must choose whether to participate in this open-label extension study.
finance.yahoo.com › news › ionis-presents-positiveIonis presents positive Phase 2 data from open label ... Nov 13, 2022 · Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) today presented positive results from a Phase 2 open-label extension (OLE) study evaluating the safety and efficacy of its investigational antisense ...
Ionis presents positive Phase 2 data from open label extension study … 13.11.2022 · Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) today presented positive results from a Phase 2 open-label extension (OLE) study evaluating the safety and efficacy of its investigational antisense ...
PDF The Safety of Deutetrabenazine for Chorea in Huntington Disease: An ... In this single-arm open-label extension study (Alterna-tives for Reducing Chorea in Huntington Disease [ARC-HD]), the safety and ecacy of deutetrabenazine were evaluated for the treatment of chorea associated with Huntington disease; compared to the pivotal double-blind study, no new safety concerns arose.
Fulcrum Therapeutics to Present New Data from the Open Label Extension ... 12.10.2022 · - 96-week findings support losmapimod as a disease-modifying therapy for FSHD - U.S. Food and Drug Administration (FDA) granted Fast Track Designation in 2021 - Industry Symposium on October 12 ...
Centessa Pharmaceuticals Announces Additional 18-Months of Continued ... After Part 2, participants were offered to continue into an open-label extension (OLE) of the Phase 2a study. OLE Period (Part 3 and Part 4): In Part 3, 22 subjects who completed Part 2 (six month repeat dose) received a flat dose of 60 mg of SerpinPC administered as a subcutaneous injection once every 4 weeks for 48 weeks.
Ionis presents positive Phase 2 data from open label extension study … 13.11.2022 · CARLSBAD, Calif., Nov. 13, 2022 /PRNewswire/ -- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) today presented positive results from a Phase 2 open-label extension (OLE) study evaluating the safety ...
Ionis presents positive Phase 2 data from open label extension study of ... carlsbad, calif., nov. 13, 2022 /prnewswire/ -- ionis pharmaceuticals, inc. (nasdaq: ions) today presented positive results from a phase 2 open-label extension (ole) study evaluating the...
markets.businessinsider.com › news › stocksIonis presents positive Phase 2 data from open label ... Nov 13, 2022 · CARLSBAD, Calif., Nov. 13, 2022 /PRNewswire/ -- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) today presented positive results from a Phase 2 open-label extension (OLE) study evaluating the safety ...
What is an open label extension study? • NCK Pharma Open-label trials may be appropriate for comparing two very similar treatments to determine which is most effective. An open-label trial may be unavoidable under some circumstances, such as comparing the effectiveness of a medication to intensive physical therapy sessions. admin Recent Industry Update What if Design of experiments (DoE) approach?
Use Extension Studies To Enhance Phase 3 Data - Clinical Leader An open-label extension study is one that will lie between a double-blind, randomized controlled drug trial (the parent trial) and the request for FDA approval. Jigna Parekh, a senior study manager for inVentiv Health Clinical, has been working in the clinical field for more than 10 years and is currently embedded with Sanofi performing ...
Consent to open label extension studies: some ethical issues A recent MEDLINE search for "open label extension studies", limited by publication type to randomised controlled trials, produced 55 references between 1992 and 2000. Open label extension studies typically follow on from (and are hence an extension of) a double blind, placebo controlled trial of a new medication.
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... There is another type of study that exists between the traditional clinical trial phases and application for approval of a new medication: an open-label extension (OLE) study, sometimes also called a long-term extension study.













![PDF] Open-label extension study following the Late-Onset ...](https://d3i71xaburhd42.cloudfront.net/772736580ed3b581db38a6826e341bb620140413/2-Figure1-1.png)











Post a Comment for "39 open-label extension study"