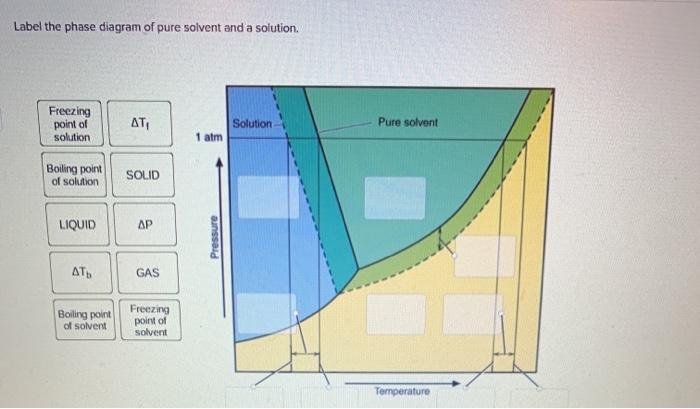
44 phase diagram of pure solvent and solution
Phase diagram of pure solvent and solution. - ResearchGate Phase diagram of pure solvent and solution. Source publication Design and Evaluation of Multiple Effect Evaporator Systems According to the Steam Ejector Position Article Full-text available Jan... Binary Phase Diagram - an overview | ScienceDirect Topics WebTHERMODYNAMICS OF SOLIDS. Milton Ohring, in Engineering Materials Science, 1995. 5.6.1.1 Substitutional Solid Solution. Binary phase diagrams usually contain an assortment of single-phase materials known as solid solutions and these have already been introduced in Sections 5.1 and 5.4.3.In the substitutional solid solution alloy the involved solute and …
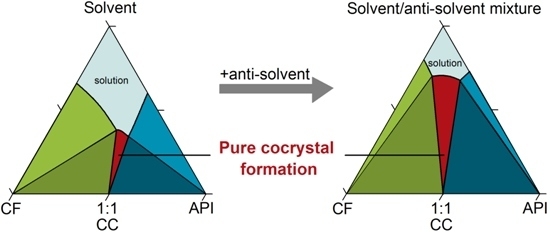
Ternary Phase Diagram - an overview | ScienceDirect Topics WebTernary phase diagrams are used to represent all possible mixtures of three solvents [1]; they are described in Chapter 3.Here, we shall indicate how they should be used to minimize the solvent consumption. Figure 2.1 (top) shows the methanol–chloroform–water ternary phase diagram with the tie-lines in the biphasic domain. Five particular compositions are …
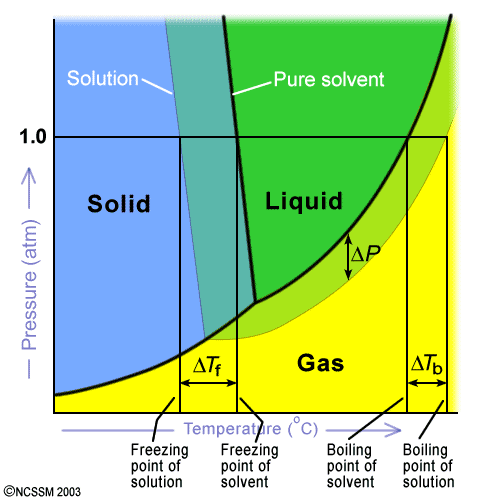
Phase diagram of pure solvent and solution
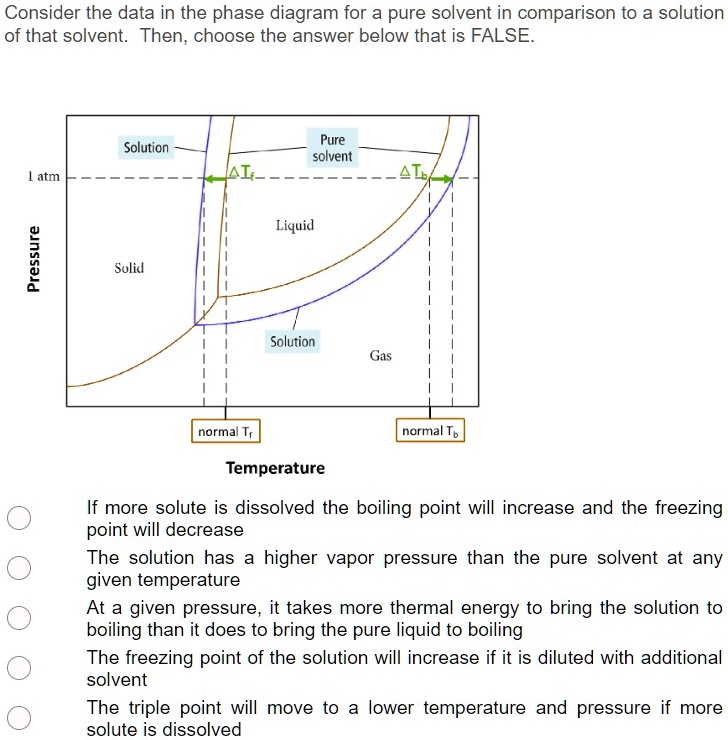
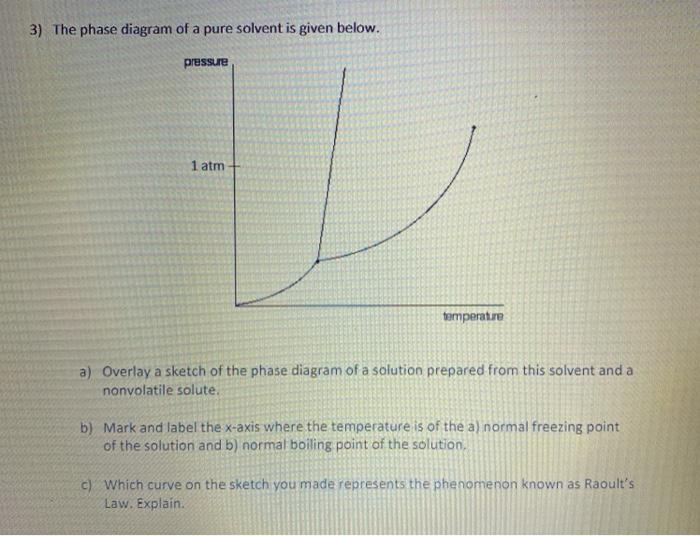
What are the most important differences between the phase di - Quizlet What are the most important differences between the phase diagram of a pure solvent and the phase diagram of a solution of that solvent? Solution Verified Create an account to view solutions By signing up, you accept Quizlet's Terms of Service and Privacy Policy Continue with Google Continue with Facebook Sign up with email Solubility Diagram - an overview | ScienceDirect Topics A typical pressure-solubility diagram corresponding to the temperature range in which a s 2 = SCF equilibrium exists (cf. Fig. 5.1) is shown in the left part of Fig. 5.3.It is important to notice the significant increase of the solubility with pressure at a temperature close to T UCEP and that no vapor-liquid equilibrium exists in this region. The influence of the pure solvent's density and ... Liquid/Solid Phase Diagram - an overview | ScienceDirect Topics The (solid + liquid) phase diagram for ( x1 n-C 6 H 14 + x2 c-C 6 H 12) has a eutectic at T = 170.59 K and x2 = 0.3317. A solid phase transition occurs in c-C 6 H 12 at T = 186.12 K, resulting in a second invariant point in the phase diagram at this temperature and x2 = 0.6115, where liquid and the two solid forms of c-C 6 H 12 are in equilibrium.
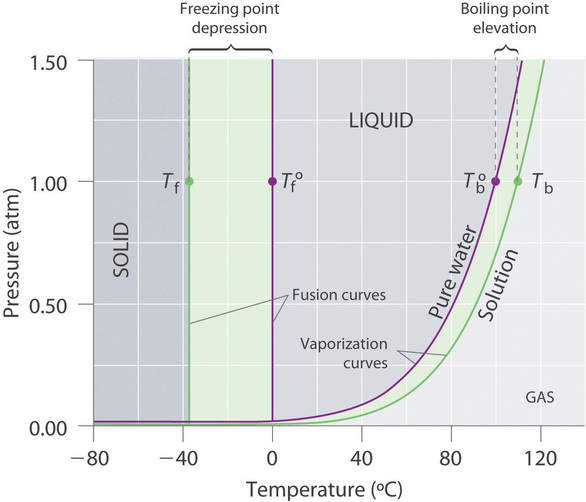
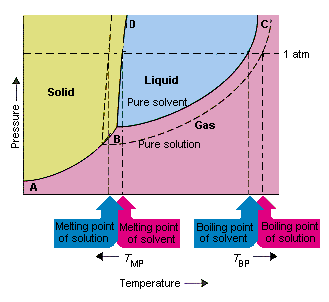
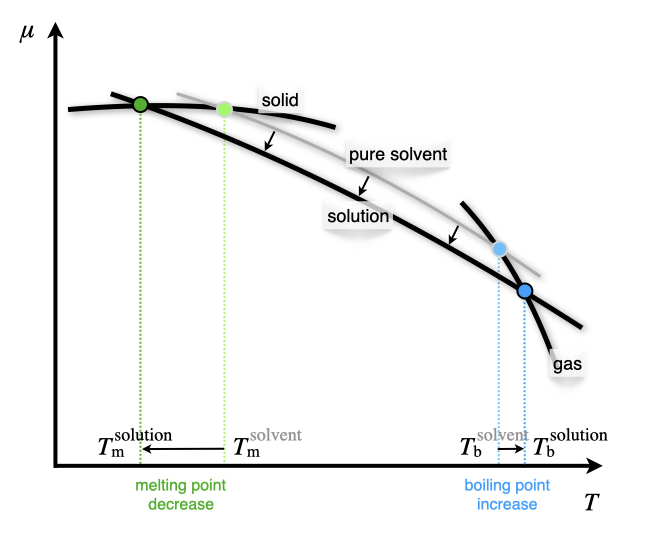
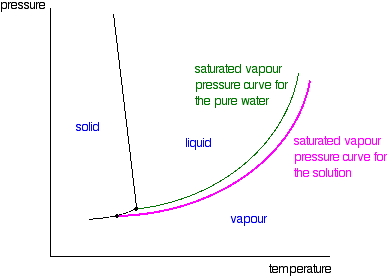
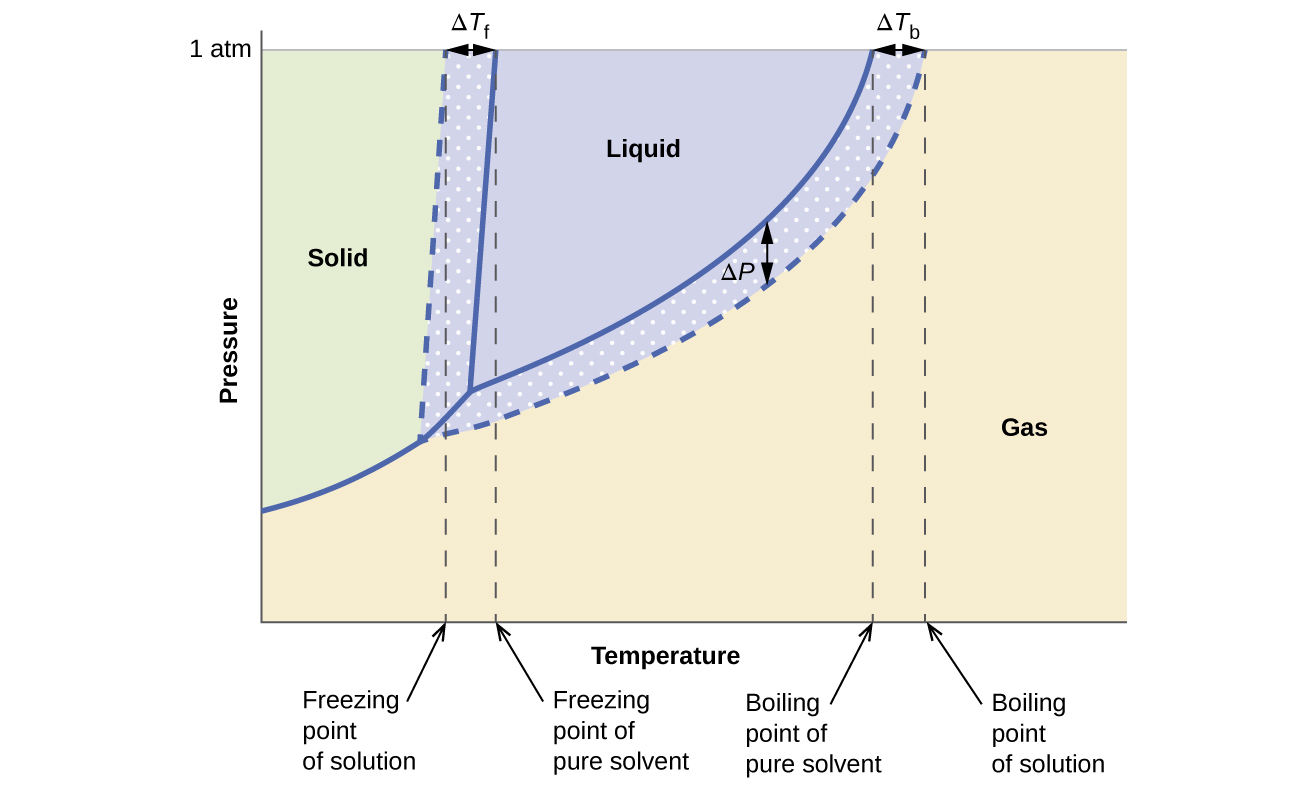
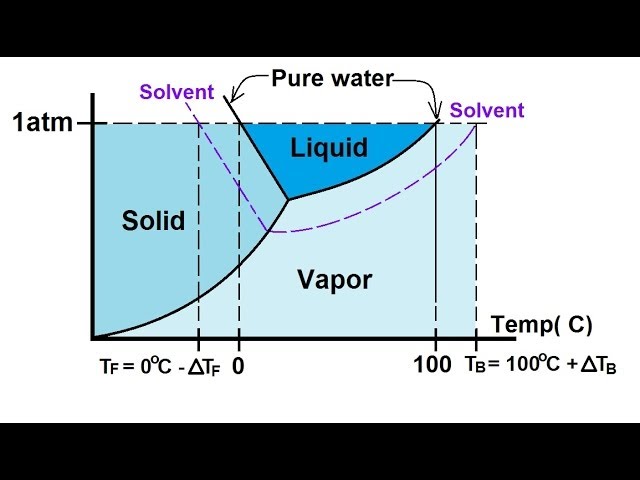
Phase diagram of pure solvent and solution. Volatile & Nonvolatile Solute Properties - Study.com Web10.01.2022 · In chemistry, a solution is made up of a solvent and a solute. The solvent is a substance, usually, a liquid, into which the solute, often a solid or a liquid, is dissolved. An example of a ... Solubility - Wikipedia WebIn chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, … The figure shows two phase diagrams, one for a pure liquid (black line ... Similarly, the normal boiling point of the pure substance at constant pressure is indicated by C, and the raised normal boiling point of the solution with the pure liquid as the solvent (again, due to addition of nonvolatile solute to the pure liquid substance) is indicated by D, since T ↑ rightwards. ΔT b = T b − T * b = iKbm Phase Diagram: Meaning and Types | Material Engineering In this case, the phase is a solid solution a of composition 90A-10B. Similarly, point 0, when dropped to the base line, will give the composition of the other phase constituting the mixture, in this case liquid solution of composition 74A-26B. ... (α + β) phase constitutes of pure copper and pure silver. iv. Below line CEG (779°C), there is ...
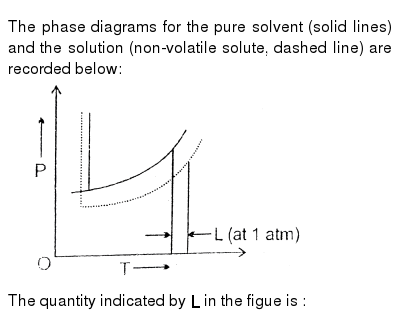
The phase diagram for solvent and solutions is shown in the figure ... The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A A B B C C D D Hard Solution Verified by Toppr Correct option is D) The normal boiling point of the solution is that temperature at which vapour pressure of solution equals to 1 atm. The phase diagrams for the pure solvent (solid lines) and the solution ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by `L` i... › ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves. The phase diagrams for the pure solvent (solid lines) and the solution ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by L in the figure is: A. `Deltap` B. `DeltaT_(f)` C. `K_(b)m` D. `K_(f)m`
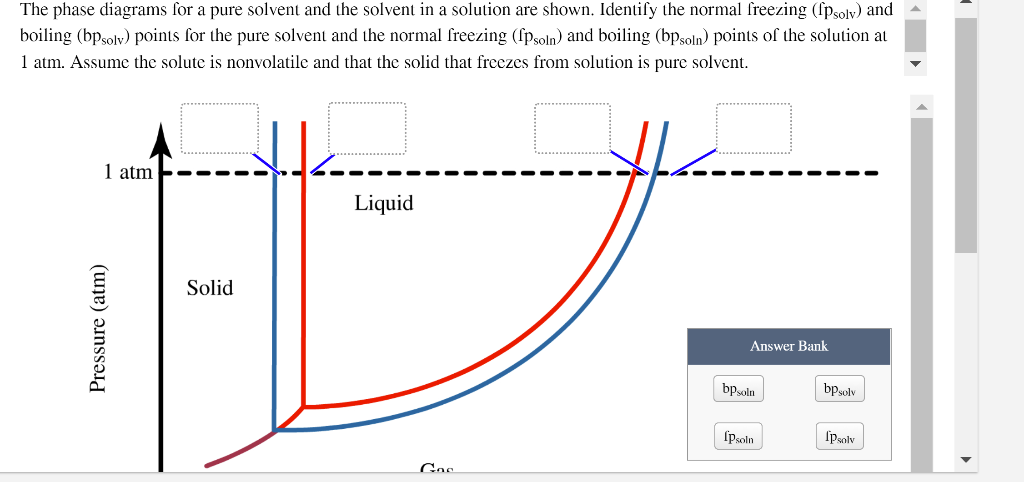
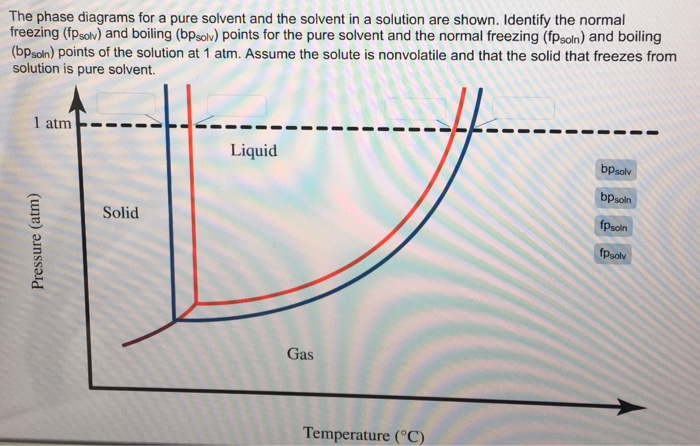
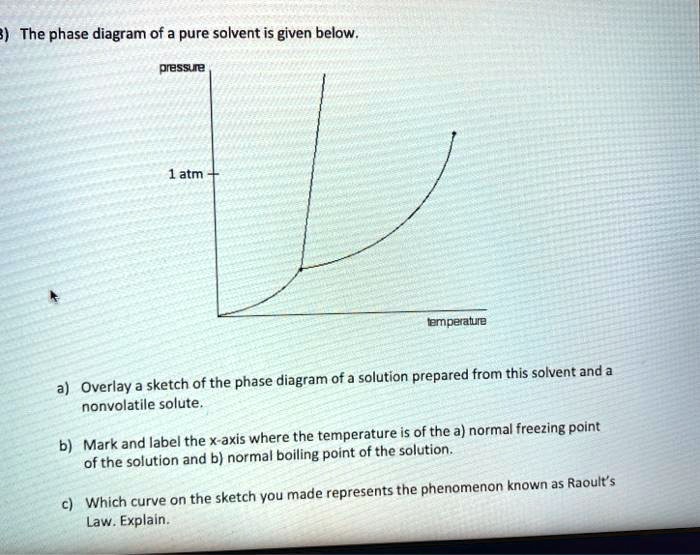
Label The Phase Diagram Of Pure Solvent And A Solution. The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal. Phase Diagrams of Water & CO2 Explained - Chemistry - Melting, Boiling & Critical Point A niko commission by vallsmaxime. Phase diagram - Wikipedia WebThe simplest phase diagrams are pressure–temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase … Mixed Solvent systems : Phasediagram In all the depicted phase diagrams, the markers represent experimental data while the solubility equilibrium lines were calculated with the Extended UNIQUAC model. Notice that the temperatures vary between the diagrams. In all the graphs below, the focus is on the lower part of the diagrams. The apex with pure K 2 SO 4 is therefore not shown. Mixture - Wikipedia WebAir is an example of a solution as well: a homogeneous mixture of gaseous nitrogen solvent, in which oxygen and smaller amounts of other gaseous solutes are dissolved. Mixtures are not limited in either their number of substances or the amounts of those substances, though in a homogeneous mixture the solute-to-solvent proportion can only …
Colloid - Wikipedia WebA colloid has a dispersed phase and a continuous phase, whereas in a solution, the solute and solvent constitute only one phase. ... When heavy metals or radionuclides form their own pure colloids, the term "eigencolloid" is used to designate pure phases, i.e., pure Tc (OH) 4, U(OH) 4, or Am(OH) 3. Colloids have been suspected for the long-range …
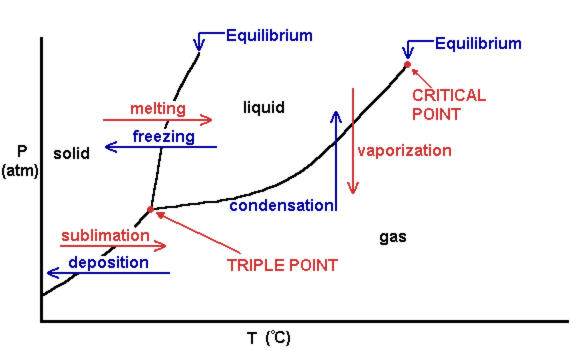
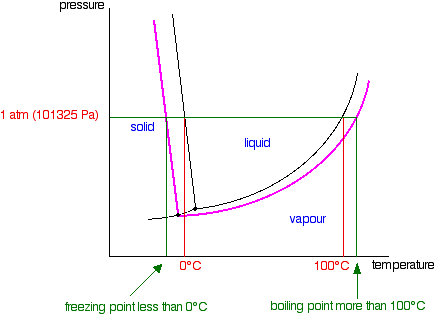
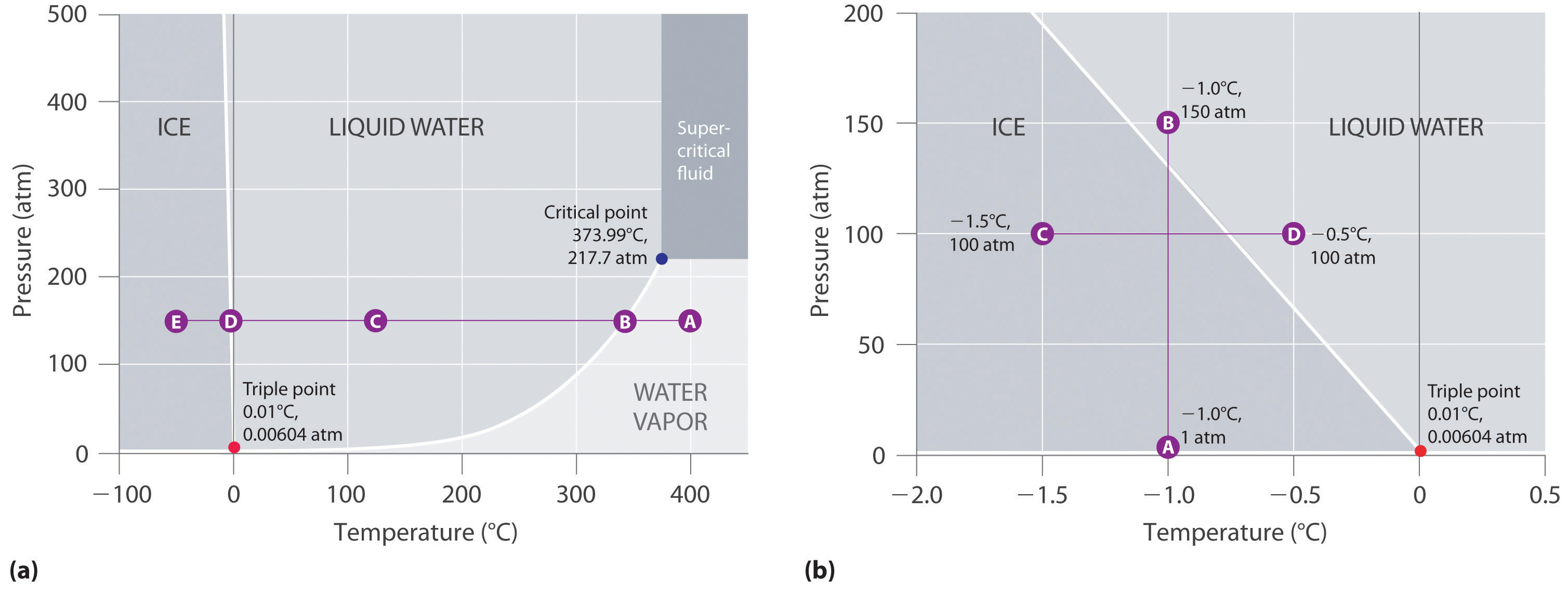
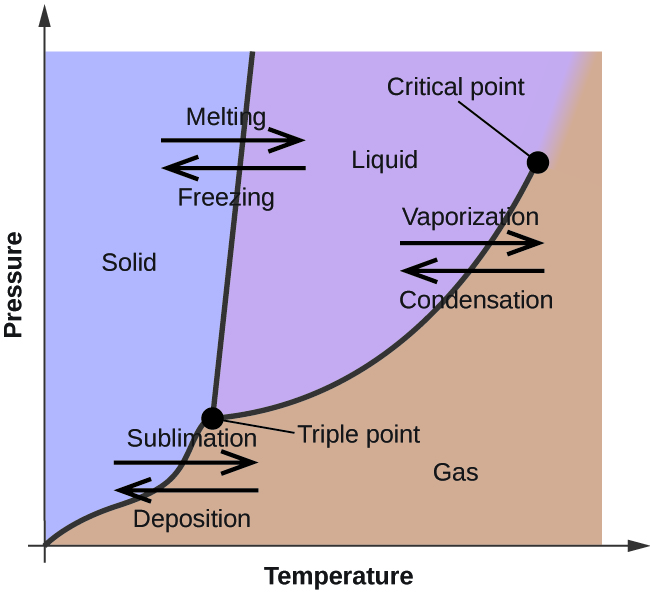
Phase Diagrams – Chemistry - University of Hawaiʻi WebAs we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water (), and a two-phase equilibrium of liquid and gaseous phases remains. At a temperature of 374 °C, the vapor pressure has risen to 218 atm, and any further increase in temperature results in the disappearance of the …
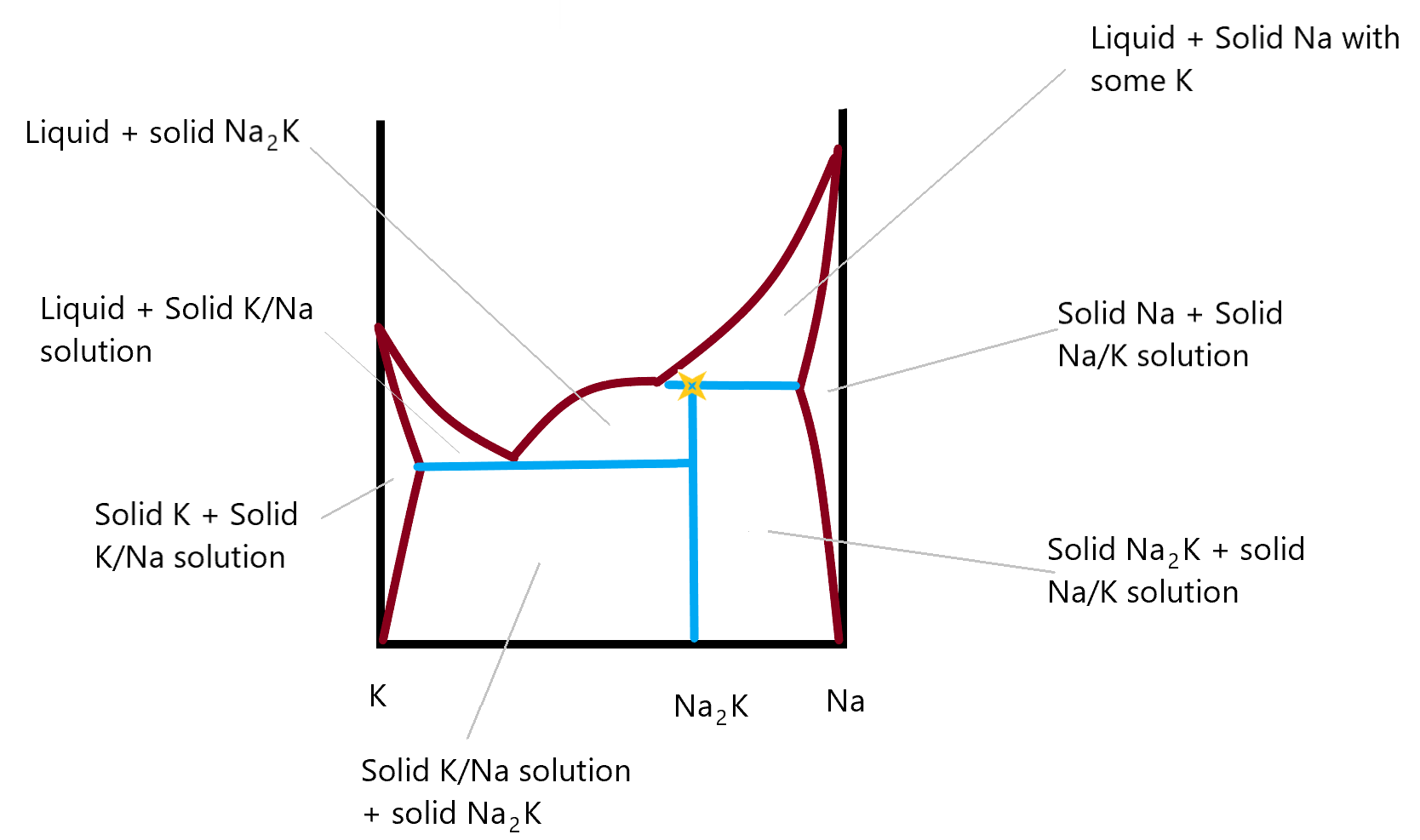
Solid solution - Wikipedia WebThe phase diagram in the above diagram displays an alloy of two metals which forms a solid solution at all relative concentrations of the two species. In this case, the pure phase of each element is of the same crystal structure, and the similar properties of the two elements allow for unbiased substitution through the full range of relative concentrations. …
Chemical potential of salt solutions : Phasediagram "Moles of solutes" is here identical to "moles of ions". The calculation of these two graphs is explained in "Electrolyte Solutions: Thermodynamics, Crystallization, Separation methods": by Kaj Thomsen. Freezing point depression of salt solutions and of ideal solution
Liquid - Wikipedia WebPure substances that are liquid under normal conditions include water, ... The phase diagram explains why liquids do not exist in space or any other vacuum. Since the pressure is zero (except on surfaces or interiors of planets and moons) water and other liquids exposed to space will either immediately boil or freeze depending on the temperature. In …
Liquid/Solid Phase Diagram - an overview | ScienceDirect Topics The (solid + liquid) phase diagram for ( x1 n-C 6 H 14 + x2 c-C 6 H 12) has a eutectic at T = 170.59 K and x2 = 0.3317. A solid phase transition occurs in c-C 6 H 12 at T = 186.12 K, resulting in a second invariant point in the phase diagram at this temperature and x2 = 0.6115, where liquid and the two solid forms of c-C 6 H 12 are in equilibrium.
Solubility Diagram - an overview | ScienceDirect Topics A typical pressure-solubility diagram corresponding to the temperature range in which a s 2 = SCF equilibrium exists (cf. Fig. 5.1) is shown in the left part of Fig. 5.3.It is important to notice the significant increase of the solubility with pressure at a temperature close to T UCEP and that no vapor-liquid equilibrium exists in this region. The influence of the pure solvent's density and ...
What are the most important differences between the phase di - Quizlet What are the most important differences between the phase diagram of a pure solvent and the phase diagram of a solution of that solvent? Solution Verified Create an account to view solutions By signing up, you accept Quizlet's Terms of Service and Privacy Policy Continue with Google Continue with Facebook Sign up with email














Post a Comment for "44 phase diagram of pure solvent and solution"